苯乙酸对甲酚酯 | 101-94-0
中文名称
苯乙酸对甲酚酯
中文别名
苯乙酸对甲苯酯;4-甲苯基苯乙酸酯;4-甲苯基苯酚乙酸酯
英文名称
p-tolyl phenylacetate
英文别名
p-cresyl phenylacetate;p-tolyl 2-phenylacetate;(4-methylphenyl) 2-phenylacetate
CAS
101-94-0
化学式
C15H14O2
mdl
——
分子量
226.275
InChiKey
OJEQSSJFSNLMLB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:74-76 °C(lit.)
-
沸点:148 °C / 1.5mmHg
-
密度:1.108±0.06 g/cm3(Predicted)
-
LogP:3.79
-
物理描述:Solid
-
溶解度:insoluble in water
-
保留指数:1790.2;1793.4;1805;1827
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:17
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.133
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
WGK Germany:2
-
RTECS号:CY1679750
-
海关编码:2916399090
-
储存条件:避光贮存于阴凉处。
SDS
p-Tolyl Phenylacetate Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: p-Tolyl Phenylacetate
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
None
Pictograms or hazard symbols
Signal word No signal word
None
Hazard statement
Precautionary statements None
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): p-Tolyl Phenylacetate
Percent: >99.0%(GC)
CAS Number: 101-94-0
Synonyms: p-Cresyl Phenylacetate , Phenylacetic Acid p-Tolyl Ester , Phenylacetic Acid p-Cresyl
Ester
Chemical Formula: C15H14O2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
p-Tolyl Phenylacetate
Section 5. FIRE-FIGHTING MEASURES
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Protective gloves.
Hand protection:
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
White - Almost white
Color:
Odor: No data available
pH: No data available
Melting point/freezing point:75 °C
Boiling Point/Range: 148 °C/0.2kPa
Flash Point: No data available
Explosive limits
Lower: No data available
No data available
Upper:
Density: No data available
No data available
Solubility:
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide
Products:
p-Tolyl Phenylacetate
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: orl-rat LD50:>5 g/kg
skn-rbt LD50:>5 g/kg
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
RTECS Number: CY1679750
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: p-Tolyl Phenylacetate
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
None
Pictograms or hazard symbols
Signal word No signal word
None
Hazard statement
Precautionary statements None
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): p-Tolyl Phenylacetate
Percent: >99.0%(GC)
CAS Number: 101-94-0
Synonyms: p-Cresyl Phenylacetate , Phenylacetic Acid p-Tolyl Ester , Phenylacetic Acid p-Cresyl
Ester
Chemical Formula: C15H14O2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
p-Tolyl Phenylacetate
Section 5. FIRE-FIGHTING MEASURES
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Protective gloves.
Hand protection:
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
White - Almost white
Color:
Odor: No data available
pH: No data available
Melting point/freezing point:75 °C
Boiling Point/Range: 148 °C/0.2kPa
Flash Point: No data available
Explosive limits
Lower: No data available
No data available
Upper:
Density: No data available
No data available
Solubility:
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide
Products:
p-Tolyl Phenylacetate
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: orl-rat LD50:>5 g/kg
skn-rbt LD50:>5 g/kg
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
RTECS Number: CY1679750
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
使用限量
FEMA(mg/kg):软饮料1.6;冷饮0.87;糖果4.8;焙烤食品5.4。此为适度使用限制(FDA §172.515,2000)。
食品添加剂最大允许使用量及残留标准 化学性质白色晶体,具有风信子和水仙花般的香气,并带有蜂蜜味。沸点为310℃,熔点为74~75℃,凝固点大于73.5℃。不溶于水和甘油,略溶于丙二醇,可溶于乙醇。
用途根据GB 2760—96的规定,苯乙酸对甲酚酯被列为允许使用的食用香料,主要用于配制果仁、蜂蜜、焦糖和奶油香精。此外,它也可用作调香剂,并可用于水仙、大花茉莉、依兰、铃兰、风信子、晚香玉、栀子等花香型的香精中。在香精中的用量一般不超过1%,偶尔也可以微量用于蜜香、奶油、坚果和焦糖等香型的食用香精。
生产方法反应信息
-
作为反应物:描述:苯乙酸对甲酚酯 在 aluminum (III) chloride 、 5%-palladium/activated carbon 、 potassium carbonate 作用下, 以 N,N-二甲基乙酰胺 、 氯苯 为溶剂, 反应 12.0h, 生成 6-methyl-3-phenyl-2-(pyridin-2-yl)-4H-chromen-4-one参考文献:名称:通过远程消除氢化物实现C–H功能化:钯催化邻酰基苯酚脱氢为类黄酮摘要:尽管人们早就知道贫电子的C–H键与碱形成碳阴离子的去质子化并广泛用于有机合成中,但从富电子的C–H键与碳阳离子或部分碳阳离子的氢化物消除是引入亲核试剂的一个重要方法。勘探面积相对较少。在这里,我们报道邻酰基苯酚的羰基β-C(sp 3)–H键氢可被分子内酚羟基取代形成O杂环,然后将O杂环脱氢成类黄酮。在不添加氧化剂和不牺牲氢受体的情况下,Pd / C催化级联反应。DOI:10.1021/acs.orglett.6b03652
-
作为产物:参考文献:名称:二酰基二硫化物:4-(N,N-二甲基氨基)吡啶催化的苯酚化学选择性酰化试剂摘要:为了通过DMAP(4-(N,N-二甲基氨基)吡啶)催化实现有效的酯形成,发现了通用且优异的酰化试剂二酰基二硫化物。该方案为酚和伯脂族羟基的位点选择性酰化提供了一个有希望的合成平台,这大大扩展了保护基化学的领域。该试剂的重要性还体现在其出色的耐湿性,高效率以及在合成化学和生物学上有意义的天然产物修饰方面的潜力。DOI:10.1021/acs.orglett.6b02818
-
作为试剂:描述:参考文献:名称:一种阿司咪唑药物中间体对氟苄胺的合成方法摘要:本发明公开了一种阿司咪唑药物中间体对氟苄胺的合成方法,通过在二氯甲烷和苯乙酸对甲酚酯混合溶液中,加入N‑(4‑氟苯甲基)邻苯二甲酰亚胺进行升温回流反应,加入亚硫酸氢钾溶液,降温,加入硝酸钠溶液,抽滤、滤液中加入2,6‑二氯‑4‑硝基苯酚溶液,用异丙醇溶液提取5‑8次,合并提取液,溴化钾溶液洗涤,脱水剂脱水,过滤,减压蒸馏,在乙腈溶液中重结晶,得晶体对氟苄胺。本发明提供的阿司咪唑药物中间体对氟苄胺的合成方法,无需通入气体,反应步骤简单许多,反应收率明显提高,同时本发明提供了一种新的合成路线,为进一步提升反应收率打下了良好的基础。公开号:CN106008229A
文献信息
-
[EN] ENCAPSULATES<br/>[FR] PRODUITS ENCAPSULÉS申请人:PROCTER & GAMBLE公开号:WO2013022949A1公开(公告)日:2013-02-14The present application relates to encapsulates, compositions, products comprising such encapsulates, and processes for making and using such encapsulates. Such encapsulates comprise a core comprising a perfume and a shell that encapsulates said core, such encapsulates may optionally comprise a parametric balancing agent, such shell comprising one or more azobenzene moieties.
-
Palladium-catalyzed carbonylation of benzylic ammonium salts to amides and esters <i>via</i> C–N bond activation作者:Weijie Yu、Shuwu Yang、Fei Xiong、Tianxiang Fan、Yan Feng、Yuanyuan Huang、Junkai Fu、Tao WangDOI:10.1039/c8ob00488a日期:——An efficient palladium-catalyzed carbonylation reaction of readily available quaternary ammonium salts with CO is reported for the first time to afford arylacetamides and arylacetic acid esters via benzylic C–N bond cleavage. This protocol features mild reaction conditions under atmospheric pressure of CO, a redox-neutral process without an additional oxidant, and a broad substrate scope for various
-
Synthesis of 7-hydroxy-6<i>H</i>-naphtho[2,3-<i>c</i>]coumarin <i>via</i> a TsOH-mediated tandem reaction作者:Ding Wang、Zhishuang Ma、Nana Wang、Chenyu Li、Tao Wang、Yong Liang、Zunting ZhangDOI:10.1039/d0cc04452k日期:——A concise and efficient method for the synthesis of 7-hydroxy-6H-naphtho[2,3-c]coumarin using available 1-(2-hydroxyphenyl)-2-phenylethanone and Meldrum's acid has been developed. This transformation involved a tandem aldol reaction/lactonization/Friedel–Crafts reaction to form a lactone ring and a benzene ring. It showed high atom economy with water and acetone as the byproducts. Mechanism studies
-
BITTER TASTE MODIFIERS INCLUDING SUBSTITUTED 1-BENZYL-3-(1-(ISOXAZOL-4-YLMETHYL)-1H-PYRAZOL-4-YL)IMIDAZOLIDINE-2,4-DIONES AND COMPOSITIONS THEREOF申请人:SENOMYX, INC.公开号:US20160376263A1公开(公告)日:2016-12-29The present invention includes compounds and compositions known to modify the perception of bitter taste, and combinations of said compositions and compounds with additional compositions, compounds, and products. Exemplary compositions comprise one or more of the following: cooling agents; inactive drug ingredients; active pharmaceutical ingredients; food additives or foodstuffs; flavorants, or flavor enhancers; food or beverage products; bitter compounds; sweeteners; bitterants; sour flavorants; salty flavorants; umami flavorants; plant or animal products; compounds known to be used in pet care products; compounds known to be used in personal care products; compounds known to be used in home products; pharmaceutical preparations; topical preparations; cannabis-derived or cannabis-related products; compounds known to be used in oral care products; beverages; scents, perfumes, or odorants; compounds known to be used in consumer products; silicone compounds; abrasives; surfactants; warming agents; smoking articles; fats, oils, or emulsions; and/or probiotic bacteria or supplements.本发明涵盖已知用于改变苦味感知的化合物和组合物,以及所述组合物和化合物与额外的组合物、化合物和产品的组合。示例组合物包括以下一种或多种:冷却剂;无活性药物成分;活性药用成分;食品添加剂或食品;调味剂或调味增强剂;食品或饮料产品;苦味化合物;甜味剂;苦味剂;酸味调味剂;咸味调味剂;鲜味调味剂;植物或动物产品;已知用于宠物护理产品中的化合物;已知用于个人护理产品中的化合物;已知用于家用产品中的化合物;制药制剂;局部制剂;大麻衍生或与大麻相关的产品;已知用于口腔护理产品中的化合物;饮料;香味、香水或除臭剂;已知用于消费品中的化合物;硅化合物;磨料;表面活性剂;发热剂;吸烟物品;脂肪、油脂或乳化剂;和/或益生菌或补充剂。
-
ORGANIC CARBONATES WITH VANILLA ODOR申请人:Gaudin Jean-Marc公开号:US20130011352A1公开(公告)日:2013-01-10The invention relates to a method to confer, enhance, improve or modify the odor properties of a perfuming composition or of a perfumed article, by adding to the composition or article an effective amount of at least a compound of formula (I) wherein R 1 represents a C 1-3 hydrocarbon group; R 2 represents a C 1-3 hydrocarbon group; and one R 3 represents a C 1-3 hydrocarbon group, and the other R 3 represents a hydrogen atom. The invention also relates to perfuming compositions and perfuming consumer products containing these compounds.该发明涉及一种方法,通过向香精组合物或带香物品中添加至少一种化合物的有效量,来赋予、增强、改善或修改香气特性。其中,所述化合物的化学式为(I),其中R1代表一个C1-3烃基;R2代表一个C1-3烃基;一个R3代表一个C1-3烃基,另一个R3代表一个氢原子。该发明还涉及含有这些化合物的香精组合物和香精消费品。
表征谱图
-
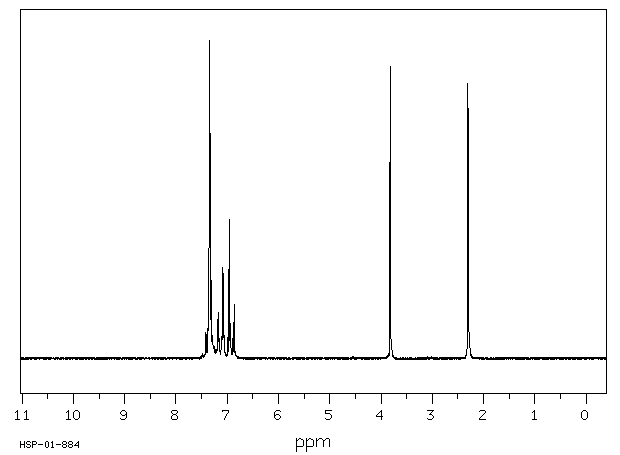
氢谱1HNMR
-
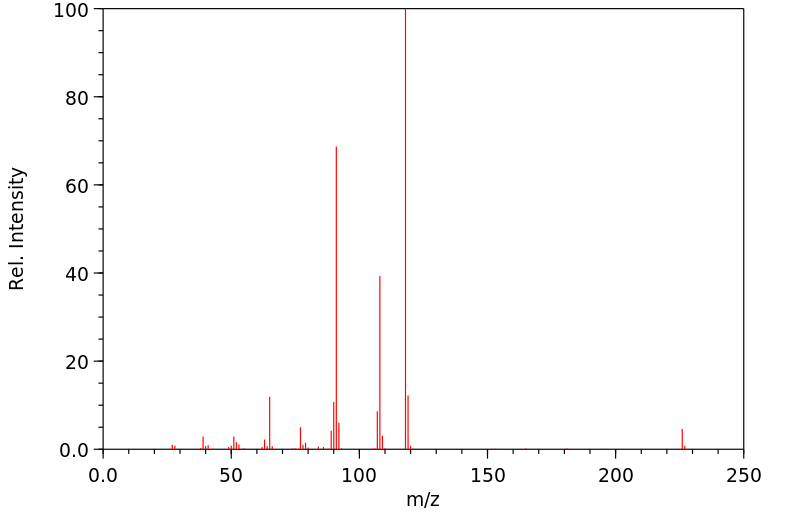
质谱MS
-
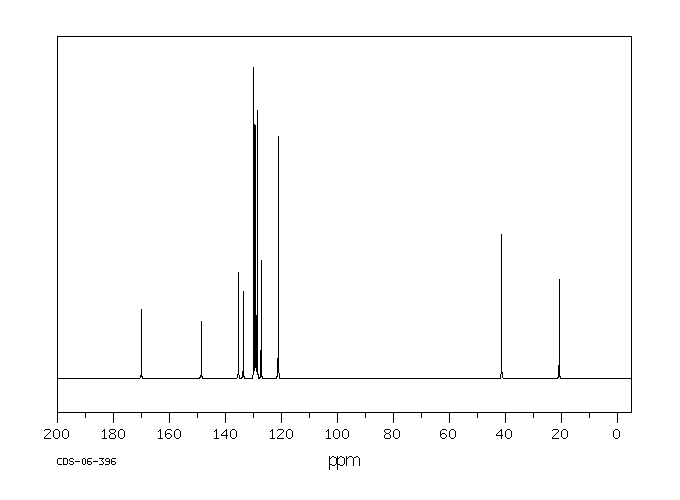
碳谱13CNMR
-
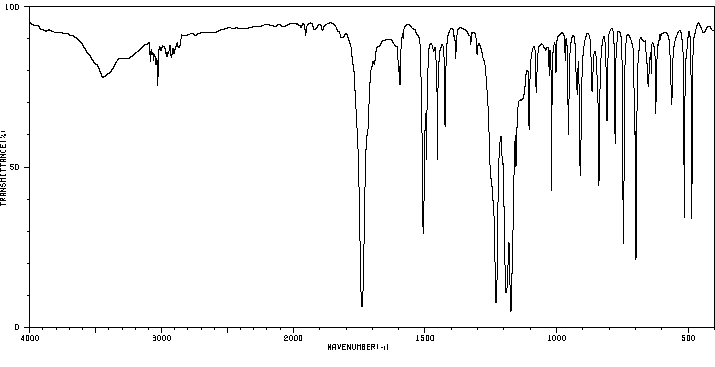
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
马来酰亚胺四聚乙二醇CH2CH2COOPFPESTER
马来酰亚胺六聚乙二醇CH2CH2COOPFPESTER
马来酰亚胺-酰胺-PEG8-四氟苯酚酯
马来酰亚胺-四聚乙二醇-五氟苯酯
马来酰亚胺-三聚乙二醇-五氟苯酚酯
靛酚乙酸酯
阿立哌唑标准品002
间硝基苯基戊酸酯
间氯苯乙酸乙酯
间乙酰苯甲酸
钾4-乙酰氧基苯磺酸酯
酚醛乙酸酯
邻苯二酚二乙酸酯
邻甲苯基环己甲酸酯
邻甲氧基苯乙酸酯
辛酸苯酯
辛酸对甲苯酚酯
辛酸五氯苯基酯
辛酸-(3-氯-苯基酯)
辛酰溴苯腈
苯酰胺,3,4-二(乙酰氧基)-N-[6-氨基-1,2,3,4-四氢-1-(4-甲氧苯基)-3-甲基-2,4-二羰基-5-嘧啶基]-
苯酚-乳酸
苯酚,4-异氰基-,乙酸酯(ester)
苯酚,4-[(四氢-2H-吡喃-2-基)氧代]-,乙酸酯
苯酚,3-(1,1-二甲基乙基)-,乙酸酯
苯酚,2-溴-3-(二溴甲基)-5-甲氧基-,乙酸酯
苯甲醇,4-(乙酰氧基)-3,5-二甲氧基-
苯甲酸,4-(乙酰氧基)-2-氟-
苯氧基氯乙酸苯酯
苯基金刚烷-1-羧酸酯
苯基氰基甲酸酯
苯基庚酸酯
苯基庚-6-炔酸酯
苯基己酸酯
苯基呋喃-2-羧酸酯
苯基吡啶-2-羧酸酯
苯基十一碳-10-烯酸酯
苯基乙醛酸酯
苯基乙酸酯-d5
苯基丙二酸单苯酯
苯基丙-2-炔酸酯
苯基丁-2,3-二烯酸酯
苯基4-乙基环己烷羧酸
苯基3-乙氧基-3-亚氨基丙酸盐
苯基2-(苯磺酰基)乙酸酯
苯基2-(4-甲氧基苯基)乙酸酯
苯基2-(2-甲氧基苯基)乙酸酯
苯基2-(2-甲基苯基)乙酸酯
苯基-乙酸-(2-甲酰基-苯基酯)
苯基-乙酸-(2-环己基-苯基酯)