2,4-二氯-α-甲基苯甲醇 | 1475-13-4
中文名称
2,4-二氯-α-甲基苯甲醇
中文别名
2,4-二氯-α-甲基苄醇;1-(2,4-二氯苯基)乙醇;2,4-二氯-Alpha-甲基苯甲醇;1-(2,4-二氯苯基)乙基醇
英文名称
1-(2,4-dichlorophenyl)ethanol
英文别名
1-(2,4-dichlorophenyl)ethan-1-ol;2,4-dichloro-α-methylbenzyl alcohol
CAS
1475-13-4
化学式
C8H8Cl2O
mdl
MFCD00004511
分子量
191.057
InChiKey
KWZDYNBHZMQRLS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:125-126 °C (7 mmHg)
-
密度:1.29
-
闪点:108 °C
-
稳定性/保质期:
如果按照规定使用和储存,则不会分解,也未有已知危险反应。请避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
海关编码:2906299090
-
危险性防范说明:P280,P302+P352,P305+P351+P338,P261
-
危险性描述:H315,H319,H335
-
储存条件:请将贮藏器密封,并存放在阴凉、干燥处。同时,确保工作环境有良好的通风或排气设施。
SDS
| Name: | 2 4-Dichloro-alpha-methylbenzyl alcohol Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 1475-13-4 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1475-13-4 | 2,4-dichloro-alpha-methylbenzyl alcoho | 100.0 | 216-019-4 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Antidote: None reported.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Clean up spills immediately, observing precautions in the Protective Equipment section. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep container tightly closed. Keep away from heat, sparks and flame.
Avoid ingestion and inhalation. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames.
Storage:
Keep away from heat, sparks, and flame. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1475-13-4: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Clear liquid
Color: colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 125 - 126 deg C @ 7.00mm Hg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 108 deg C ( 226.40 deg F)
Explosion Limits, lower: N/A
Explosion Limits, upper: N/A
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.2930g/cm3
Molecular Formula: C8H8Cl2O
Molecular Weight: 191.06
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents, acids, acid anhydrides, acid chlorides.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, hydrogen fluoride gas.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1475-13-4 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2,4-dichloro-alpha-methylbenzyl alcohol - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Other No information available.
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 1475-13-4: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1475-13-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1475-13-4 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 (2,4-二氯苯基)环氧乙烷 2-(2,4-dichlorophenyl)oxirane 13692-15-4 C8H6Cl2O 189.041 —— (1-(2,4-dichlorophenyl)ethoxy)trimethylsilane —— C11H16Cl2OSi 263.239 2,4-二氯苯乙酮 2,4-dichloroacetophenone 2234-16-4 C8H6Cl2O 189.041 1-(1-溴乙基)-2,4-二氯苯 1-(1-bromoethyl)-2,4-dichlorobenzene 20444-01-3 C8H7BrCl2 253.954 2,4-二氯-1-(1-氯乙基)苯 2,4-dichloro-1-(1-chloroethyl)benzene 60907-89-3 C8H7Cl3 209.503 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (S)-1-(2,4-二氯苯基)乙醇 (S)-1-(2,4-dichlorophenyl)ethanol 179237-92-4 C8H8Cl2O 191.057 —— (1-(2,4-dichlorophenyl)ethoxy)trimethylsilane —— C11H16Cl2OSi 263.239 2,4-二氯苯乙酮 2,4-dichloroacetophenone 2234-16-4 C8H6Cl2O 189.041 1-(1-溴乙基)-2,4-二氯苯 1-(1-bromoethyl)-2,4-dichlorobenzene 20444-01-3 C8H7BrCl2 253.954 2,4-二氯-1-(1-氯乙基)苯 2,4-dichloro-1-(1-chloroethyl)benzene 60907-89-3 C8H7Cl3 209.503
反应信息
-
作为反应物:描述:2,4-二氯-α-甲基苯甲醇 在 palladium on activated charcoal sodium hydroxide 、 2-己醇 、 Geotrichum candidum IFO 4597 cells on BL-100 polymer 、 氢气 、 环己酮 作用下, 以 乙醇 、 正己烷 为溶剂, 反应 48.0h, 生成 (R)-(+)-1-苯基乙醇参考文献:名称:Stereoselective oxidation and reduction by immobilized Geotrichum candidum in an organic solvent摘要:将真菌地霉菌(Geotrichum candidum)的细胞固定在水吸收性聚合物上,并用于在有机溶剂中以环己酮、环戊醇或烷-2-醇作为添加剂进行立体选择性氧化和还原反应。通过立体选择性氧化外消旋1-芳基乙醇,获得了对映体纯的(R)-1-芳基乙醇,而通过对相应的酮进行还原,获得了对映体纯的(S)-1-芳基乙醇,这与自由细胞在水中的还原反应不同,后者仅产生低ee值的(R)-或(S)-1-芳基乙醇。通过测量细胞固定在聚合物周围的有机相和水分相中底物和产物的分布,研究了反应机制。使用氘代化合物来确定添加剂的作用。DOI:10.1039/a900936a
-
作为产物:描述:2,4-二氯苯乙酮 在 Ru(II) aminophosphine sodium isopropylate 作用下, 以 异丙醇 为溶剂, 反应 48.0h, 以99%的产率得到2,4-二氯-α-甲基苯甲醇参考文献:名称:半三明治Ru(II)氨基膦配合物催化的氢转移摘要:某些含Ru(II)配合物的合成与催化研究。氨基膦 配体 N,N-二甲基-2-二苯基膦乙胺(PN),光学纯的(R C,S pl)-2- {1-(N,N-二甲基氨基)乙基} -1-二苯基膦二茂铁(PPFA)和N,N-二甲基-2-二苯基膦基苯胺描述了(DBD),[RuCp(CH 3 CN)3 ] +与它们反应配体得到阳离子络合物[RuCp(PN-κ Ñ,κ P)(CH 3 CN)] +(1A),[(小号钌,[R Ç,小号PL)-RuCp(PPFA-κ Ñ,κ P)( CH 3 CN)] +(1B),和[RuCp(DBD-κ ñ,κ P )(CH 3 CN)] +(1C),分别以高收率。由此,残留的CH 3 CN配体可以被Br取代-在加入净的4溴在CH 2氯2,从而在中性络合物RuCp(PN)Br(上形成图2a),(小号的Ru,- [R Ç,小号PL)-RuCp(PPFA) Br(2b)和RuCpDOI:10.1039/b104128m
文献信息
-
An air and moisture tolerant iminotrihydroquinoline-ruthenium(<scp>ii</scp>) catalyst for the transfer hydrogenation of ketones作者:Jiaoyan Li、Yingmiao Ma、Zheng Wang、Qingbin Liu、Gregory A. Solan、Yanping Ma、Wen-Hua SunDOI:10.1039/c8dt01919c日期:——with RuCl2(PPh3)3 at room temperature affords the ruthenium(II) chelate (8-NH2-C9H10N)RuCl2(PPh3)2 (E), in which the two triphenylphosphine ligands are disposed mutually cis. By contrast, when the reaction is performed at reflux ligand oxidation/dehydrogenation occurs along with cis–trans reorganization of the triphenylphosphines to form the 8-imino-5,6,7-trihydroquinoline-ruthenium(II) complex, (88-氨基-5,6,7,8-四氢喹啉在室温下与RuCl 2(PPh 3)3反应得到钌(II)螯合物(8-NH 2 -C 9 H 10 N)RuCl 2(PPh 3)2(E),其中两个三苯基膦配体相互顺式排列。相反,当反应在回流条件下进行时,配体会发生氧化/脱氢以及三苯基膦的顺式-反式重组,形成8-亚氨基-5,6,7-三氢喹啉-钌(II)络合物,(8-NH C9 H 9 N)RuCl 2(PPh 3) 2( F)。通过单独加热E溶液至回流,也可以高收率获得配合物F。它们的分子结构比较突出了F中的二齿亚胺配体比E中的含胺对应物优越的结合性能。两种络合物在多种烷基-,芳基-和环烷基的酮的转移氢化中非常有效,从而提供其相应的仲醇,其负载量低至0.1 mol%。明显地, F即使在与空气接触的反应釜中,即使是台式质量的2-丙醇也可以提供出色的转化率,而E的催化效率会因空气的存在而降低,但只能在惰性条件下有效地操作。
-
Aza-crown compounds synthesised by the self-condensation of 2-amino-benzyl alcohol over a pincer ruthenium catalyst and applied in the transfer hydrogenation of ketones作者:Shanshan Zhang、Zheng Wang、Qianrong Cao、Erlin Yue、Qingbin Liu、Yanping Ma、Tongling Liang、Wen-Hua SunDOI:10.1039/d0dt03257c日期:——conformation structure of 3. These aza-crown compounds have been explored to study ferric initiation of transfer hydrogenation (TH) of ketones into their corresponding secondary alcohols in the presence of 2-propanol with a basic t-BuOK solution, achieving a high conversion (up to 95%) by a ferric complex with 2 in a low loading (0.05 mol%).
-
Looking for new antiplasmodial quinazolines: DMAP-catalyzed synthesis of 4-benzyloxy- and 4-aryloxy-2-trichloromethylquinazolines and their in vitro evaluation toward Plasmodium falciparum作者:Armand Gellis、Nicolas Primas、Sébastien Hutter、Gilles Lanzada、Vincent Remusat、Pierre Verhaeghe、Patrice Vanelle、Nadine AzasDOI:10.1016/j.ejmech.2016.04.059日期:2016.8A DMAP catalyzed synthesis of new 4-benzyloxy- and 4-aryloxy-2-trichloromethylquinazolines was studied, in a view to react 4-chloroquinazolines with poorly nucleophilic alcohols such as benzylic alcohols, via a simple and cheap SNAr reaction approach. A fast (1 h) general operating procedure, affording good reaction yields, was achieved under microwave irradiation. Thus, a series of 35 molecules was
-
Ruthenium ONO-Type Pincer Complex: Synthesis, Structural Characterization, and Catalysis作者:Yao Zhang、Xingwei Li、Soon Hyeok HongDOI:10.1002/adsc.201000083日期:——A novel nitrone‐based pincer ligand was developed by a single‐step synthesis from N‐(tert‐butyl)hydroxylamine acetate and 2,6‐pyridinedicarboxaldehyde. The developed ligand allowed us to synthesize a cationic ruthenium pincer complex. A distorted octahedral coordination environment around the ruthenium center was observed. The complex showed excellent catalytic activity in transfer hydrogenation reactions
-
A Comparative Study on Asymmetric Reduction of Ketones Using the Growing and Resting Cells of Marine-Derived Fungi作者:Hui Liu、Bi-Shuang Chen、Fayene de Souza、Lan LiuDOI:10.3390/md16020062日期:——performance involves resting cells rather than growing cell biotransformation, which is one-step process that benefits from the simultaneous growth and biotransformation, eliminating the need for catalysts preparation. In this paper, asymmetric reduction of 14 aromatic ketones to the corresponding enantiomerically pure alcohols was successfully conducted using the growing and resting cells of marine-derived fungi
表征谱图
-
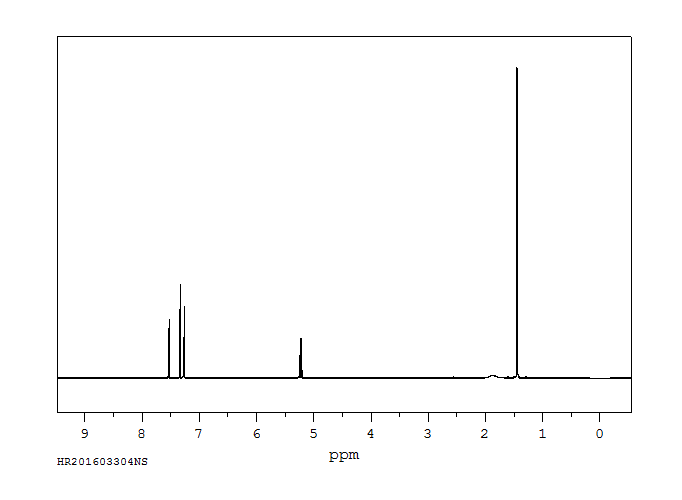
氢谱1HNMR
-
质谱MS
-
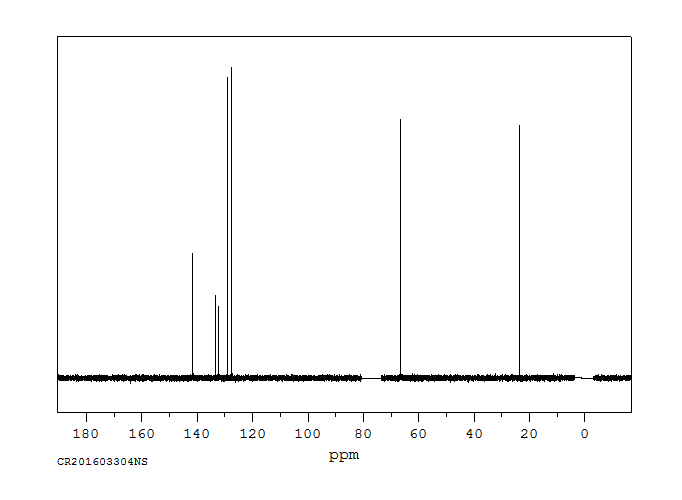
碳谱13CNMR
-
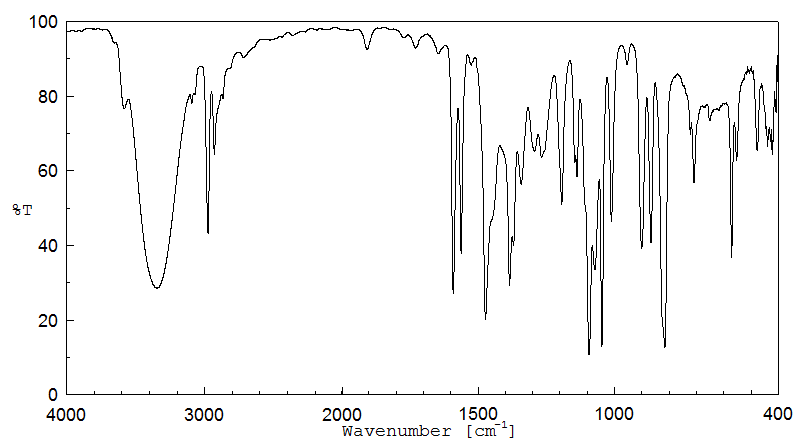
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫