3-(4-氯苯基)-1,1-二甲基硫脲 | 2212-17-1
中文名称
3-(4-氯苯基)-1,1-二甲基硫脲
中文别名
——
英文名称
N,N-dimethyl-N'-p-chlorophenylthiourea
英文别名
N'-(4-chloro-phenyl)-N,N-dimethyl-thiourea;N'-(4-Chlor-phenyl)-N,N-dimethyl-thioharnstoff;3-(4-chlorophenyl)-1,1-dimethyl-2-thiourea;1.1-Dimethyl-3-<4-chlor-phenyl>-thioharnstoff;N,N-Dimethyl-N'-p-chlorphenyl-thioharnstoff;N'-(4-Chlorophenyl)-N,N-dimethylthiourea;3-(4-chlorophenyl)-1,1-dimethylthiourea
CAS
2212-17-1
化学式
C9H11ClN2S
mdl
MFCD00018585
分子量
214.719
InChiKey
FWGDAHLBEXSAPT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:150-152 °C
-
沸点:283.3±42.0 °C(Predicted)
-
密度:1.292±0.06 g/cm3(Predicted)
-
溶解度:>32.2 [ug/mL]
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:13
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.222
-
拓扑面积:47.4
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2930909090
SDS
反应信息
-
作为反应物:描述:3-(4-氯苯基)-1,1-二甲基硫脲 在 吡啶 、 碘苯二乙酸 、 nickel dibromide 作用下, 以 1,4-二氧六环 为溶剂, 反应 0.17h, 以86%的产率得到6-氯-N,N-二甲基-2-苯并噻唑胺参考文献:名称:通过镍催化的分子内氧化CH官能团轻度合成2-取代的苯并噻唑。摘要:已经报道了从芳基硫脲开始制备2-氨基苯并噻唑的高效合成方法。通过使用镍催化剂,芳基硫脲进行分子内氧化CH键官能化,以良好至优异的产率得到所需的2-氨基苯并噻唑。该方案具有廉价的催化剂,催化剂用量低,反应条件温和,反应时间短,收率良好至优异的特点,并且可以轻松按比例放大至克级,而收率几乎没有下降。DOI:10.1021/acs.joc.9b02543
-
作为产物:描述:参考文献:名称:A Convenient Route to Substituted Thiocarbamides摘要:A variety of substituted thioureas result from thiuram disufides by a simple reaction with different amines.DOI:10.1080/00397919508013859
文献信息
-
Synthesis and Pesticidal Properties of Thio and Seleno Analogs of Some Common Urea Herbicides作者:Jerzy Zakrzewski、Maria KrawczykDOI:10.1080/10426500802391692日期:2009.7.13Thio and seleno analogs of fenuron, isoproturon, chlorotoluron, metoxuron, monuron, and diuron were synthesized from the corresponding aryl amines. Their reaction with thiophosgene leads to isothiocyanates. Aryl amines were also converted (via isocyanides) to isoselenocyanates. The reaction of both isothio- and isoselenocyanates with dimethylamine affords the corresponding thio and seleno analogs of
-
Facile synthesis of S -arylisothioureas via the S -arylation of arylthioureas with aryl iodides in water作者:Xing Liu、Hui Zhu、Shi-Bo Zhang、Yu Cheng、Han-Ying Peng、Zhi-Bing DongDOI:10.1016/j.tetlet.2018.07.012日期:2018.8An environmentally benign, simple and highly efficient protocol for the synthesis of S-arylisothiourea derivatives has been achieved in good to excellent yields by reacting a series of aryl iodides with arylthioureas, using inexpensive CuSO4·5H2O as catalyst in water without PTC (phase transfer catalyst). The protocol features easy performance, good to excellent yields, good tolerance towards a variety
-
An Efficient Chan-Lam <i>S</i> -Arylation of Arylthioureas with Arylboronic Acids作者:Xing Liu、Shi-Bo Zhang、Hui Zhu、Yu Cheng、Han-Ying Peng、Zhi-Bing DongDOI:10.1002/ejoc.201800892日期:2018.8.31A convenient and efficient protocol has been developed for the preparation of aryl-isothioureas based on the Chan-Lam C-S coupling reaction. The desired aryl-isothioureas were synthesized in good yields (up to 95%) in the presence of Cu(OAc)(2)H2O as catalyst and bipyridine as ligand. A variety of functional groups on the aryl-thiourea and arylboronic acid reagents are tolerated. The method features
-
Insertion of Arynes into Thioureas: A New Amidine Synthesis作者:Kallolmay Biswas、Michael F. GreaneyDOI:10.1021/ol202049v日期:2011.9.16thioureas via a formal π-insertion into the C═S bond. The reaction contrasts with that of ureas, which proceeds via benzyne σ-insertion into the C–N bond, and represents a new, operationally simple route to functionalized amidines.
-
Copper-Catalyzed and Air-Mediated Mild Cross-Dehydrogenative Coupling of Aryl Thioureas and Dialkyl<i>H</i>-Phosphonates: The Synthesis of Thiophosphonates作者:Cai-Zhu Chang、Xing Liu、Hui Zhu、Li Wu、Zhi-Bing DongDOI:10.1021/acs.joc.8b02011日期:2018.11.2A copper-catalyzed and air-mediated mild cross-dehydrogenative coupling of aryl thioureas and dialkyl H-phosphonates to produce thiophosphonates has been reported. Without addition of base or acid, air as an oxidant, the phosphorylation occurred smoothly to give the target products in moderate to excellent yields at room temperature. The protocol allows the direct formation of a sulfur–phosphorus bond
表征谱图
-
氢谱1HNMR
-
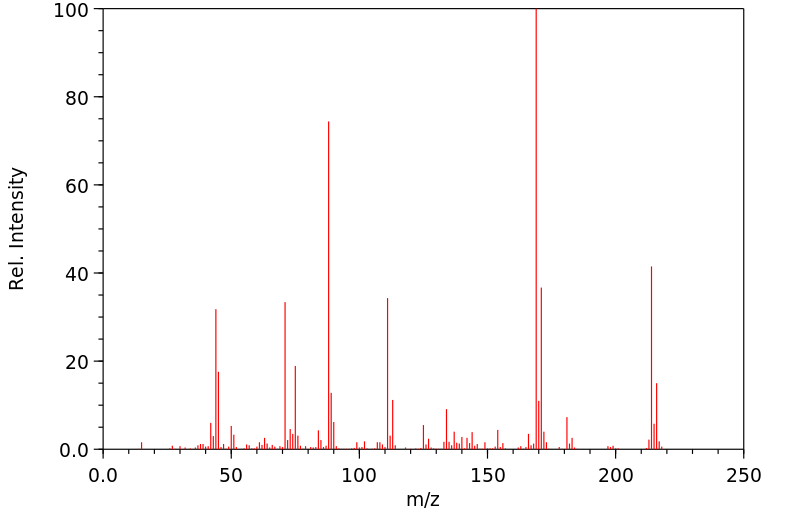
质谱MS
-
碳谱13CNMR
-
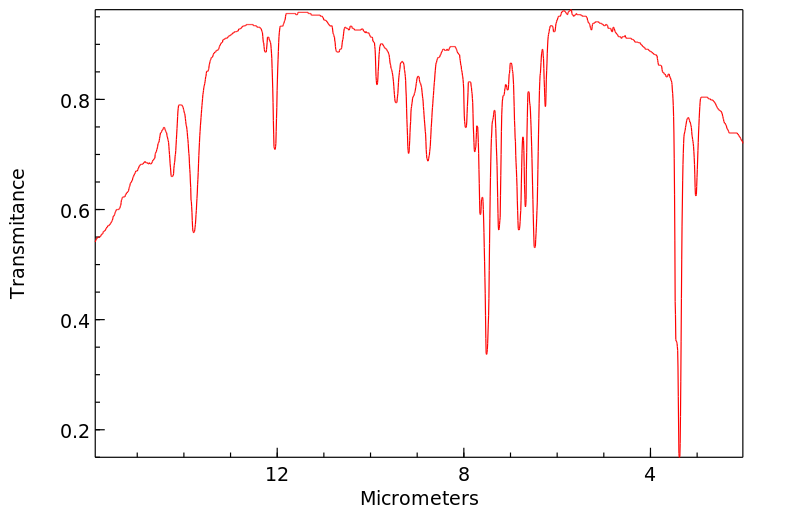
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫