1,20-二溴二十烷 | 14296-16-3
中文名称
1,20-二溴二十烷
中文别名
——
英文名称
1,20-dibromoeicosane
英文别名
1,20-dibromoicosane
CAS
14296-16-3
化学式
C20H40Br2
mdl
——
分子量
440.346
InChiKey
AYGKJDVBWSEQHQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:408.98°C (rough estimate)
-
密度:1.2131 (estimate)
计算性质
-
辛醇/水分配系数(LogP):10.8
-
重原子数:22
-
可旋转键数:19
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2903399090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,10-二溴癸烷 1,10-dibromodecane 4101-68-2 C10H20Br2 300.077 1,6-二溴己烷 1 ,6-dibromohexane 629-03-8 C6H12Br2 243.969 11-溴-1-十一烯 1-undecen-11-ylbromide 7766-50-9 C11H21Br 233.192 1,5-二溴戊烷 1,5-dibromo-pentane 111-24-0 C5H10Br2 229.942 6-溴正己醇 1-bromo-6-hexanol 4286-55-9 C6H13BrO 181.073 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,38-dibromooctatriacontane 102436-01-1 C38H76Br2 692.829 —— 20-bromo-eicos-1-ene 811794-49-7 C20H39Br 359.434 20-溴-1-二十烷醇 20-bromoeicosane-1-ol 92002-48-7 C20H41BrO 377.449 —— 38-bromooctatriacont-1-ene 1315070-40-6 C38H75Br 611.917
反应信息
-
作为反应物:描述:参考文献:名称:Ring-closure reactions. 7. Kinetics and activation parameters of lactone formation in the range of 3- to 23-membered rings摘要:DOI:10.1021/ja00450a031
-
作为产物:描述:二十烷二酸二甲酯 在 N-溴代丁二酰亚胺(NBS) 、 lithium aluminium tetrahydride 、 三苯基膦 作用下, 以 四氢呋喃 为溶剂, 反应 1.0h, 生成 1,20-二溴二十烷参考文献:名称:针对冈比亚曲霉乙酰胆碱酯酶的半胱氨酸靶向杀虫剂既不是选择性抑制剂也不是可逆抑制剂。摘要:针对疟疾媒介冈比亚按蚊和其他蚊子的针对乙酰胆碱酯酶半胱氨酸的杀虫剂已经被引入。我们已经将烯烃复分解用于制备高产率的以半胱氨酸为目标的杀虫剂。在冈比亚按蚊和人乙酰胆碱酯酶上对制备的具有琥珀酰亚胺或马来酰亚胺部分的化合物进行了评估,两种酶均具有较高的不可逆抑制性,但选择性较差。半胱氨酸结合的概念没有通过几种方法得到证实,并且在水/缓冲液环境中观察到所选最有效/选择性化合物的稳定性差。因此,我们的发现不支持所制备的一系列琥珀酰亚胺或马来酰亚胺化合物的半胱氨酸靶向选择性杀虫剂的概念。DOI:10.1021/acsmedchemlett.9b00477
文献信息
-
Synthesis of fluorescent probes for localized membrane fluidity measurements作者:Alain Beck、Denis Heissler、Guy DuportailDOI:10.1016/s0040-4020(01)86422-x日期:1991.1The synthesis of two long-chain homologues of the fluorescent membrane probes TMA-DPH and DPHpPC is described. The long-chain phosphatidylcholine could be synthesized from palmitoyl-lysophosphatidylcholine and 21-(diphenylhexatrienyl) henicosanoic acid only when the promoting agent was 1,1′-thionyldiimidazole.
-
.beta.1-Selective adrenoceptor antagonists. 1. Synthesis and .beta.-adrenergic blocking activity of a series of binary (aryloxy)propanolamines作者:R. W. Kierstead、A. Faraone、F. Mennona、J. Mullin、R. W. Guthrie、H. Crowley、B. Simko、L. C. BlaberDOI:10.1021/jm00365a004日期:1983.11A series of binary (aryloxy)propanolamines has been prepared and examined in vitro and in vivo for beta-adrenoreceptor blocking activity. These symmetrical compounds consist of two (S)-(phenyloxy)propanolamine pharmacophores coupled through alkylenedioxy or poly(oxyethylenedioxy) linking units of varying lengths. Examples of such binary compounds linked through the 2,2', 3,3', and 4,4' positions in
-
THE ELECTROLYSIS OF ω-BROMOCARBOXYLIC ACIDS作者:R.G. WoolfordDOI:10.1139/v62-280日期:1962.9.1
The first successful preparation of ω,ω′-dibromides from the electrolyses of a series of ω-bromocarboxylic acids, Br(CH2)nCOOH (n = 5 to n = 11), is reported. Under conditions of fairly low temperature and current density, yields from 54 to 71% of these dibromides were obtained.The electrolysis of 11-bromoundecanoic acid is discussed as an example of how a small change in experimental conditions can produce a considerable change in the products of reaction. At 50°, the product was mainly 1,20-dibromoeicosane, whereas at 65° the products were mainly the esters methyl 11-bromoundecanoate and methyl 11-methoxyundecanoate.
-
Synthesis of Alkylene-Bridged Diphenyl-Oligoynes作者:Christian Klinger、Otto Vostrowsky、Andreas HirschDOI:10.1002/ejoc.200500851日期:2006.3Alkylene chains with up to 40 methylene groups were employed. The two terminal acetylene functions were introduced prior to the oligoyne elongation by twofold introduction of additional C2-acetylene or C4-butadiyne building blocks. The final step was the intramolecular acetylene coupling upon which very large macrocycles with up to 62 ring members containing segregated sp-, sp2- and sp3-segments were formed
-
Bright White-Light Emission from a Single Organic Compound in the Solid State作者:Qing-Yuan Yang、Jean-Marie LehnDOI:10.1002/anie.201400155日期:2014.4.25White‐light‐emitting materials and devices have attracted enormous interest because of their great potential for various lighting applications. We herein describe the light‐emitting properties of a series of new difunctional organic molecules of remarkably simple structure consisting of two terminal 4‐pyridone push–pull subunits separated by a polymethylene chain. They were found to emit almost “pure”发白光的材料和设备因其在各种照明应用中的巨大潜力而引起了人们的极大兴趣。我们在这里描述了一系列结构非常简单的新型双功能有机分子的发光特性,这些分子由两个末端的4-吡啶酮推挽亚基组成,这些亚单位被一条多亚甲基链隔开。发现它们作为固态的单一有机化合物以及掺入聚合物膜中时几乎发出“纯”白光。据我们所知,它们是迄今为止报道的最简单的发白光有机分子。
表征谱图
-
氢谱1HNMR
-
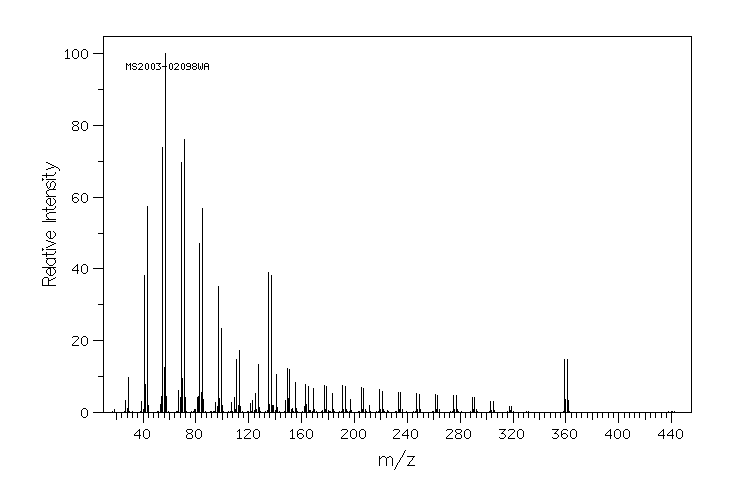
质谱MS
-
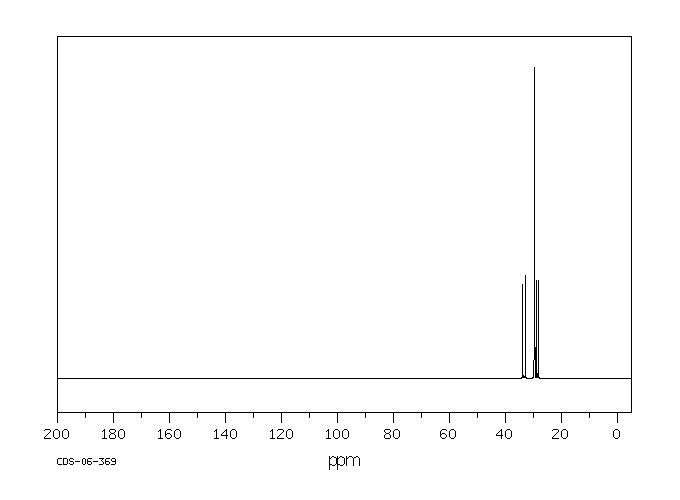
碳谱13CNMR
-
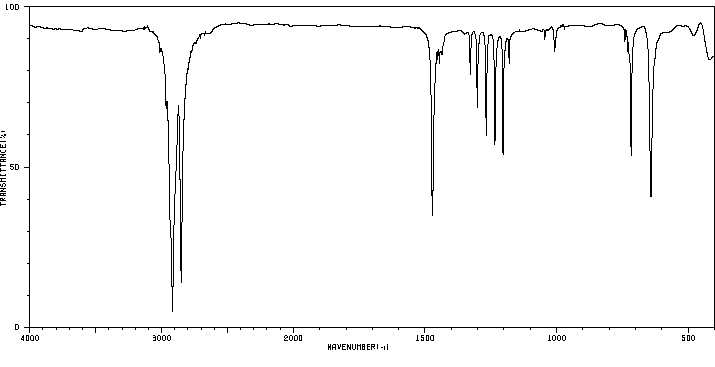
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(3-溴-1-丙炔-1-基)环丙烷
马杜拉霉素
顺-3,顺-6-1-溴壬二烯
顺,反,顺-1,2,3,4-四(2-溴乙基)环丁烷
金刚烷-2,2-d2
辛烷,1,5-二溴-
苯并噻唑,6-异硫氰酸根合5-甲基-(9CI)
苯(甲)醛,3-甲氧基-4-硝基-
硬脂基溴
硫杂二溴化
癸基溴
甲基环丙基溴化镁
环戊醇1-乙基-3-(苯甲基)-(9CI)
环戊烯-1,3-溴-(7CI,9CI)
环丙烷,1-溴-1-(3,3-二甲基-1-丁炔基)-2,2-二甲基-
环丁基溴
溴甲基环戊烷
溴甲基环己烷
溴甲基环丙烷
溴甲基环丁烷
溴甲基
溴环戊烷-D9
溴己烷-D3
溴己烷
溴化环辛基甲基
溴代环辛烷
溴代环戊烷
溴代环庚烷
溴代环丙烷
溴代异辛烷
溴代异丁烷
溴代叔丁烷-D9
溴代叔丁烷
溴代十四烷-D29
溴代十四烷
溴代十六烷-D33
溴代十六烷
溴代十五烷
溴代十二烷
溴代二十烷
溴乙醛
溴乙烷-D3
溴乙烷-D1
溴乙烷-2-13C
溴乙烷-13C2
溴乙烷-1-13C
溴乙烷-1,1-d2
溴乙烷-1,1,2,2-d4
溴乙烷
溴丙烷-D4