bis-(4-chlorophenyl) thionocarbonate | 67803-58-1
中文名称
——
中文别名
——
英文名称
bis-(4-chlorophenyl) thionocarbonate
英文别名
bis(4-chlorophenyl) thionocarbonate;thiocarbonic acid O,O'-bis-(4-chloro-phenyl ester);Thiokohlensaeure-O,O'-bis-(4-chlor-phenylester);thiocarbonic acid bis(4-chloro-phenyl) ester;carbonothioic acid, O,O-bis(4-chlorophenyl) ester;bis(4-chlorophenoxy)methanethione
CAS
67803-58-1
化学式
C13H8Cl2O2S
mdl
——
分子量
299.177
InChiKey
FPCLKEOKECTRRI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):5.5
-
重原子数:18
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:50.6
-
氢给体数:0
-
氢受体数:3
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-氯苯基氯硫代甲酸酯 4-chlorophenyl chlorothionoformate 937-64-4 C7H4Cl2OS 207.08 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 双(4-氯苯基)碳酸酯 bis(4-chlorophenyl) carbonate 2167-53-5 C13H8Cl2O3 283.111
反应信息
-
作为反应物:描述:参考文献:名称:Cussans, Nigel J.; Ley, Steven V.; Barton, Derek H. R., Journal of the Chemical Society. Perkin transactions I, 1980, p. 1650 - 1653摘要:DOI:
-
作为产物:描述:参考文献:名称:A Study of the Schönberg Rearrangement of Diaryl Thioncarbonates to Diaryl Thiolcarbonates1摘要:DOI:10.1021/ja01614a035
文献信息
-
Kinetic Study of the Phenolysis of Bis(4-nitrophenyl) Carbonate, Bis(4-nitrophenyl) Thionocarbonate, and Methyl 4-Nitrophenyl Thionocarbonate作者:Enrique A. Castro、Mauricio Angel、David Arellano、José G. SantosDOI:10.1021/jo0101252日期:2001.10.1The reactions of a homogeneous series of phenols with bis(4-nitrophenyl) carbonate (BNPC), bis(4-nitrophenyl) thionocarbonate (BNPTOC), and methyl 4-nitrophenyl thionocarbonate (MNPTOC) are subjected to a kinetic investigation in water, at 25.0 degrees C and ionic strength of 0.2 M (KCl). Under excess of phenol over the substrate, all the reactions obey pseudo-first-order kinetics and are first order均相系列苯酚与碳酸双(4-硝基苯酯),双(4-硝基苯基)硫代碳酸酯(BNPTOC)和4-硝基苯基硫代碳酸甲酯(MNPTOC)的反应在水中进行25.0摄氏度,离子强度为0.2 M(KCl)。在底物上过量的苯酚下,所有反应均遵循拟一级反应动力学,并且在酚盐阴离子中为一级反应。BNPC的反应显示出线性的Bönsted型图,斜率β= 0.66,与一致的机理一致(一个步骤)。相比之下,BNPTOC和MNPTOC的双相布朗斯台德图在高pK(a)时斜率分别为β= 0.30和0.44,在低pK(a)时斜率分别为β= 1.25和1.60,与逐步一致机制。对于两种硫代碳酸酯的反应,布朗斯台德图中心(pK(a)(0))的pK(a)值为7.1,它对应于4-硝基苯酚的pK(a)。这证实了硫代碳酸酯的酚类是逐步过程,形成了阴离子四面体中间体。通过比较标题反应和相似反应的动力学和机理,可以得出以下结论:(i)在阴离子四
-
Kinetic and theoretical study on nucleofugality in the phenolysis of 3-nitrophenyl and 4-nitrophenyl 4-cyanophenyl thionocarbonates作者:Enrique A. Castro、Alvaro Cañete、Paola R. Campodónico、Marjorie Cepeda、Paulina Pavez、Renato Contreras、José G. SantosDOI:10.1016/j.cplett.2013.04.002日期:2013.5evaluate the nucleofugality of the corresponding leaving groups. For the reaction of 2 only 4-nitrophenoxide is obtained as leaving group. For the reaction of 1 the nucleofugality ratio 3-nitrophenoxide/4-cyanophenoxide is 1/3 from the corresponding T− intermediate. Theoretical calculations confirm the experimental results. From these results it can be concluded that the non-leaving group affects the
-
The invention of radical reactions. Part XXIV. Relative rates of acylation and radical deoxygenation of secondary alcohols.作者:Derek H.R. Barton、Joseph Dorchak、Joseph Cs. JaszberenyiDOI:10.1016/s0040-4020(01)90359-x日期:1992.9Secondary alcohols were transformed into various thiocarbonyl derivatives. Reduction of these compounds using tributyltin hydride and an initiator afforded the corresponding deoxy-compounds. Half-life and competitive measurements showed that all these reactions were fast and could be run to completion.
-
The Schönberg Rearrangement. Kinetics of the Rearrangement of Bis-(4-chlorophenyl) Thioncarbonate to the Thiolcarbonate<sup>1</sup>作者:Donald H. Powers、D. Stanley TarbellDOI:10.1021/ja01582a021日期:1956.1
-
Cristofoli, Walter A.; Benn, Michael, Journal of the Chemical Society. Perkin transactions I, 1991, # 8, p. 1825 - 1831作者:Cristofoli, Walter A.、Benn, MichaelDOI:——日期:——
表征谱图
-
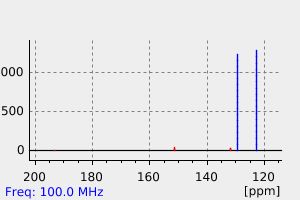
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
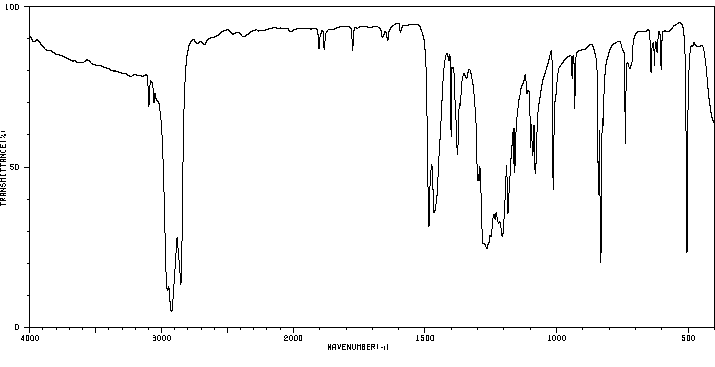
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫