13H-indeno[1,2-l]phenanthren-13-one | 86853-96-5
中文名称
——
中文别名
——
英文名称
13H-indeno[1,2-l]phenanthren-13-one
英文别名
9H-indeno[1,2-l]phenanthren-9-one;9H-indeno[2,1-l]phenanthren-9-one;indeno[1,2-l]phenanthren-13-one;indeno[1,2-l]phenanthren-13-one;Indeno[1,2-l]phenanthren-13-on;Indeno<1,2-l>phenanthren-13-on;13H-Indeno(2,1-l)phenanthren-13-one;pentacyclo[12.7.0.02,7.08,13.015,20]henicosa-1(14),2,4,6,8,10,12,15,17,19-decaen-21-one
CAS
86853-96-5
化学式
C21H12O
mdl
——
分子量
280.326
InChiKey
BAOGBBGACLJBCP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:186-187 °C
-
沸点:520.9±17.0 °C(Predicted)
-
密度:1.315±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):5.6
-
重原子数:22
-
可旋转键数:0
-
环数:5.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,2,3,4-二苯并芴 13H-indeno[1,2-l]phenanthrene 201-65-0 C21H14 266.342 —— 2-(phenanthren-9-yl)benzaldehyde 898821-54-0 C21H14O 282.342 9-甲基-10-苯基菲 9-methyl-10-phenylphenanthrene 33498-62-3 C21H16 268.358
反应信息
-
作为产物:描述:fluoren-9-yl-phenyl ketone 在 lithium aluminium tetrahydride 、 sodium dichromate 、 乙醚 、 sodium methylate 、 phosphorus pentoxide 、 xylene 作用下, 生成 13H-indeno[1,2-l]phenanthren-13-one参考文献:名称:芴的脂肪族化学:第 I 部分. 9-苯甲酰芴的衍生物摘要:已经制备了 9-苯甲酰芴的几种 C-烷基化产物。9-Methyl-9-benzoylfluorene 已转化为 9-methyl-10-phenylphenanthrene、9-phenylphenan-threne-10- 羧酸和 1,2:3,4-dibenzfluorenone.Phenyl-9-allyl-9 -芴基甲醇一步环化并重排为 14-甲基-13,14-二氢二苯并[a,c]菲。苯基-9-甲基-9-芴基甲基氯化物容易分解为9-甲基-10-苯基-菲时单独加热或在甲酸中加热,但与银或醋酸汞反应生成未重排的醋酸盐。DOI:10.1139/v59-294
文献信息
-
Benziodoxole Triflate as a Versatile Reagent for Iodo(III)cyclization of Alkynes作者:Bin Wu、Junliang Wu、Naohiko YoshikaiDOI:10.1002/asia.201701530日期:2017.12.14Hyperactive: A benziodoxole triflate promotes iodo(III)cyclization of alkynes tethered to a variety of nucleophilic moieties, affording benziodoxole-appended (hetero)arenes such as benzofurans, benzothiophenes, isocoumarins, indoles, and polyaromatics. These unprecedented (hetero)aryl-IIII compounds are easy to purify, air- and thermally stable, and amenable to various synthetic transformations.
-
Efficient Synthesis of Fluoren-9-ones by the Palladium-Catalyzed Annulation of Arynes by 2-Haloarenecarboxaldehydes作者:Jesse P. Waldo、Xiaoxia Zhang、Feng Shi、Richard C. LarockDOI:10.1021/jo8009215日期:2008.9.1Fluoren-9-ones and derivatives are readily prepared in good yields by the annulation of in situ generated arynes by 2-haloarenecarboxaldehydes in the presence of a palladium catalyst.
-
Synthesis of fluorenones viaquaternary ammonium salt-promoted intramolecular dehydrogenative arylation of aldehydes作者:Zhuangzhi Shi、Frank GloriusDOI:10.1039/c2sc21823b日期:——Biologically interesting fluorenone, xanthone and anthrone derivatives have been prepared via an intramolecular oxidative acylation process. This novel direct acylation reaction proceeded without the aid of any transition metals, acids or bases, and uses a catalytic amount of a quaternary ammonium salt in the presence of a persulfate oxidant. Initial mechanistic studies have been carried out to elucidate
-
Palladium-catalyzed cyclocarbonylation of cyclic diaryliodoniums: Synthesis of fluorenones作者:Li Liu、Jian Qiang、Shuhua Bai、Yang Li、Chunbao Miao、Jian LiDOI:10.1002/aoc.3817日期:2017.12An efficient approach to the synthesis of fluorenones via the palladium‐catalyzed cyclocarbonylation of cyclic diaryliodoniums was developed. Our route enables facile access to fluorenones with various substituents in modest to high yields.
-
Synthesis of Fluoren-9-ones by the Palladium-Catalyzed Cyclocarbonylation of <i>o</i>-Halobiaryls作者:Marino A. Campo、Richard C. LarockDOI:10.1021/jo020140m日期:2002.8.1The synthesis of various substituted fluoren-9-ones has been accomplished by the palladium-catalyzed cyclocarbonylation of o-halobiaryls. The cyclocarbonylation of 4'-substituted 2-iodobiphenyls produces very high yields of 2-substituted fluoren-9-ones bearing either electron-donating or electron-withdrawing substituents. 3'-Substituted 2-iodobiphenyls afford 3-substituted fluoren-9-ones in excellent
表征谱图
-
氢谱1HNMR
-
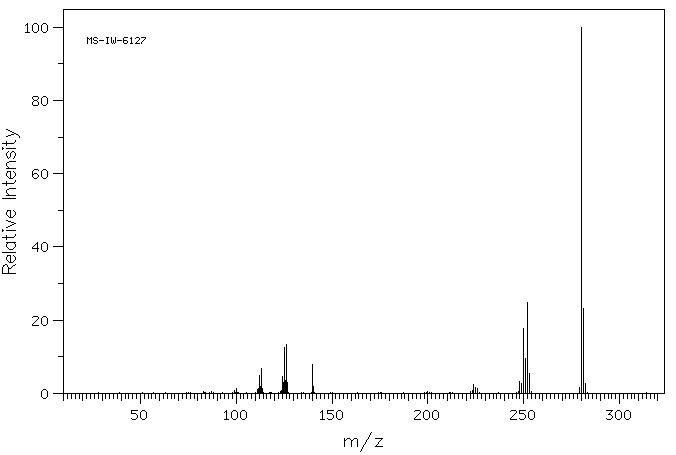
质谱MS
-
碳谱13CNMR
-
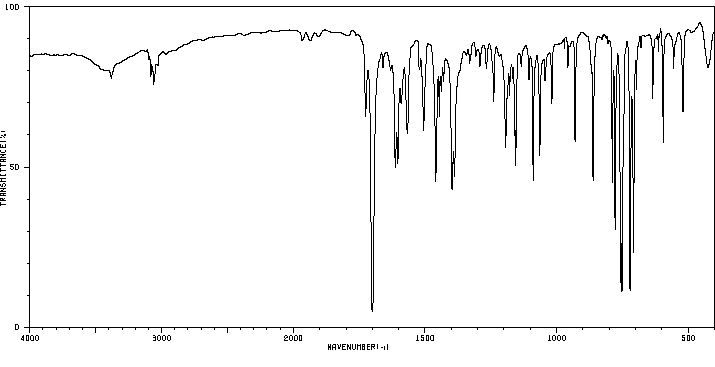
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-2,2'',3,3''-四氢-6,6''-二-9-菲基-1,1''-螺双[1H-茚]-7,7''-二醇
(6,6)-苯基-C61己酸甲酯
高雌二醇
马兜铃酸钠
马兜铃酸盐
马兜铃酸C
马兜铃酸B
马兜铃酸(1:1MIXTUREOFARISTOLOCHICACIDIANDARISTOLOCHICACIDII)
马兜铃酸 Ia
马兜铃酸 IVa
马兜铃酸
颜料黑32
颜料红179
颜料红178
颜料红149
颜料红123
顺式-菲-1,2-二醇-3,4-环氧化物
顺式-苯并(a)屈-11,12-二醇-13,14-环氧化物
雷公藤酚A
镁二(1,4,5,6,7,16,17,18,19,19,20,20-十二氯六环[14.2.1.14,7.02,15.03,8.09,14]二十-5,9,11,13,17-五烯-11-磺酸酯)
钩大青酮
钩大青酮
钙(2+)12-羟基十八烷酸酯
酒石酸布托诺啡
那布扶林
还原红32
足球烯
贝那他汀B
贝母兰素
萘并[2,3-b]荧蒽
萘并[2,1-e][1]苯并二硫杂环戊烷
萘并[2,1-C:7,8-C']二菲
萘并[1,2-e][2]苯并呋喃-1,3-二酮
萘并[1,2-b]屈
萘并[1,2-a]蒽
萘并[1,2-B]菲-6-醇
萘二(六氯环戊二烯)加合物
萘,8-溴-1,2,3-三(1,1-二甲基乙基)-6-甲基-
菲醌单缩氨基硫脲
菲醌
菲并[9,10]呋喃
菲并[9,10-e]醋菲烯
菲并[4,5-bcd]噻吩
菲并[4,5-bcd]呋喃-3-醇
菲并[4,3-d]-1,3-二噁唑-5-羧酸,10-羟基-9-甲氧基-6-硝基-
菲并[3,2-b]噻吩
菲并[2,1-d]噻唑
菲并[2'',1'',10'':4,5,6;7'',8'',9'':4',5',6']二异喹啉并[2,1-a:2',1'-a']二萘嵌间二氮杂苯-8,13-二酮
菲并(3,4-b)噻吩
菲并(1,2-b)噻吩