反,反-1,4-均二苯乙烯 | 1608-41-9
中文名称
反,反-1,4-均二苯乙烯
中文别名
trans,trans-1,4-均二苯乙烯;1,4-双(反-2-苯基乙基)苯;1,4-双(反-2-苯基乙基)苯,97%
英文名称
1,4-Distyrylbenzene
英文别名
1,4-Bis[(E)-2-phenylethenyl]benzene
CAS
1608-41-9
化学式
C22H18
mdl
——
分子量
282.385
InChiKey
IJAAWBHHXIWAHM-PHEQNACWSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:264-266℃
-
沸点:443.6±30.0 °C(Predicted)
-
密度:1.103±0.06 g/cm3(Predicted)
-
最大波长(λmax):356nm(Benzene)(lit.)
计算性质
-
辛醇/水分配系数(LogP):7.1
-
重原子数:22
-
可旋转键数:4
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
储存条件:室温
SDS
trans,trans-1,4-Distyrylbenzene
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: trans,trans-1,4-Distyrylbenzene
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: trans,trans-1,4-Distyrylbenzene
Percent: >98.0%(GC)
CAS Number: 1608-41-9
Chemical Formula: C22H18
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
trans,trans-1,4-Distyrylbenzene
Section 5. FIRE-FIGHTING MEASURES
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: Very pale yellow - Pale yellow green
Odour: No data available
pH: No data available
Melting point/freezing point:266°C
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
trans,trans-1,4-Distyrylbenzene
Section 10. STABILITY AND REACTIVITY
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
trans,trans-1,4-Distyrylbenzene
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: trans,trans-1,4-Distyrylbenzene
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: trans,trans-1,4-Distyrylbenzene
Percent: >98.0%(GC)
CAS Number: 1608-41-9
Chemical Formula: C22H18
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
trans,trans-1,4-Distyrylbenzene
Section 5. FIRE-FIGHTING MEASURES
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: Very pale yellow - Pale yellow green
Odour: No data available
pH: No data available
Melting point/freezing point:266°C
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
trans,trans-1,4-Distyrylbenzene
Section 10. STABILITY AND REACTIVITY
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
trans,trans-1,4-Distyrylbenzene
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 1-((E)-styryl)-4-((Z)-styryl)benzene 16726-72-0 C22H18 282.385 反式-1,2-二苯乙烯 (E)-1,2-diphenyl-ethene 103-30-0 C14H12 180.249 4-二苯乙烯甲醛 4-styrylbenzaldehyde 32555-96-7 C15H12O 208.26 对二乙烯苯 1,4-Divinylbenzene 105-06-6 C10H10 130.189 1-(4-氯苯基)-2-苯基乙烯 (E)-1-(4-chlorophenyl)-2-phenylethene 1657-50-7 C14H11Cl 214.694 4-溴-反-二苯乙烯 (E)-4-bromostilbene 13041-70-8 C14H11Br 259.145 —— (E,E)-1,4-bis(β-bromovinyl)benzene 64984-68-5 C10H8Br2 287.982 苯乙烯 styrene 100-42-5 C8H8 104.152 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-((E)-styryl)-4-((Z)-styryl)benzene 16726-72-0 C22H18 282.385 —— (cis,cis)-1,4-distyrylbenzene 1608-40-8 C22H18 282.385 —— 4-<β-Phenaethyl>-1-styryl-benzol 95166-77-1 C22H20 284.401
反应信息
-
作为反应物:描述:参考文献:名称:Iodine in Dimethyl Sulfoxide as a New General Reagent for the Preparative Oxidation of 1,2-Diarylethenes and 1,2-Diarylethynes to Aromatic 1,2-Diketones摘要:DOI:10.1055/s-1991-26397
-
作为产物:描述:四乙基-1,4-苯二膦酸酯 在 palladium diacetate 三甲基溴硅烷 、 十二/十四烷基二甲基氧化胺 、 四丁基氟化铵 作用下, 以 四氢呋喃 、 1,4-二氧六环 、 乙腈 为溶剂, 反应 29.5h, 生成 反,反-1,4-均二苯乙烯参考文献:名称:涉及芳基膦酸碳-磷键断裂的氧化 Heck 型反应摘要:碳-磷键的裂解是在钯催化下在利用芳基膦酸的氧化 Heck 型反应中实现的。芳基膦酸与烯烃的反应在三甲胺氧化物作为氧化剂的 TBAF 存在下以良好的收率提供芳基化产物。DOI:10.1021/ja026758v
文献信息
-
Facile Regio- and Stereoselective Hydrometalation of Alkynes with a Combination of Carboxylic Acids and Group 10 Transition Metal Complexes: Selective Hydrogenation of Alkynes with Formic Acid作者:Ruwei Shen、Tieqiao Chen、Yalei Zhao、Renhua Qiu、Yongbo Zhou、Shuangfeng Yin、Xiangbo Wang、Midori Goto、Li-Biao HanDOI:10.1021/ja2069246日期:2011.10.26highly stereo- and regioselective hydrometalation of alkynes generating alkenylmetal complex is disclosed for the first time from a reaction of alkyne, carboxylic acid, and a zerovalent group 10 transition metal complex M(PEt(3))(4) (M = Ni, Pd, Pt). A mechanistic study showed that the hydrometalation does not proceed via the reaction of alkyne with a hydridometal generated by the protonation of a
-
Palladium-Catalyzed Reductive Coupling Reaction of Terminal Alkynes with Aryl Iodides Utilizing Hafnocene Difluoride as a Hafnium Hydride Precursor Leading to <i>trans</i> -Alkenes作者:Keita Takahashi、Yohei Ogiwara、Norio SakaiDOI:10.1002/asia.201701775日期:2018.4.4Herein, we describe a reductive cross‐coupling of alkynes and aryl iodides by using a novel catalytic system composed of a catalytic amount of palladium dichloride and a promoter precursor, hafnocene difluoride (Cp2HfF2, Cp=cyclopentadienyl anion), in the presence of a mild reducing reagent, a hydrosilane, leading to a one‐pot preparation of trans‐alkenes. In this process, a series of coupling reactions
-
Fulvenes and thermochromic ethylenes. Part LXXXIII. ‘Double dibenzotropones’ within C<sub>6</sub>-C<sub>7</sub>-C<sub>6</sub>-C<sub>7</sub>-C<sub>6</sub>ring systems. Synthesis of bisbenzo[4,5]cyclohepta[1,2-a:2′,1′-d]benzene-5,7-dione and bisbenzo-[4,5]cyclohepta[1,2-a:1′,2′-d]benzene-5,13-dione作者:Israel Agranat、David AvnirDOI:10.1039/p19740001155日期:——Condensation of pyromellitic anhydride with phenylacetic acid (2 mol. equiv.) gave the two isomeric di-γ-lactones of 4,6-bis-(α-hydroxystyryl)isophthalic acid (IX) and 2,5-bis-(α-hydroxystyryl)terephthalic acid (X) in the ratio of 5:3. 4,6-Bis-(2-phenylethyl)isophthalic acid (XI) and 2,5-bis-(2-phenylethyl)terephthalic acid (XIII), formed by reduction of (IX) and (X), respectively, have been cyclized
-
Palladium-Catalyzed Stereoselective Synthesis of (E)-Stilbenes via Organozinc Reagents and Carbonyl Compounds作者:Jin-Xian Wang、Kehu Wang、Lianbiao Zhao、Hongxia Li、Ying Fu、Yulai HuDOI:10.1002/adsc.200606016日期:——In the presence of a catalytic amount of PdCl2(PPh3)2 and a silylating agent, organozinc halides reacted with carbonyl compounds to give the corresponding (E)-stilbenes in good to excellent yields under mild conditions. The reaction mechanism is briefly discussed.
-
Phenylenevinylene oligomers by Mizoroki-Heck cross coupling reaction. Structural and optoelectronic characterization作者:Sandra E. Estrada、Cristian Ochoa-Puentes、Cesar A. SierraDOI:10.1016/j.molstruc.2016.12.032日期:2017.4spectroscopies. The results showed that, with only one exception, the ED and EW groups at the ends of OPV systems lead to a bathochromic shift. This effect is intensified with the introduction of methoxy groups on the central ring. Consistent with these, the HOMO-LUMO gaps (ΔE) decreases as the strength of ED and EW substituents increases. The ED and EW substituents also lead to a decrease in the Φ f values摘要 为了研究分子结构对全反式亚苯基亚乙烯基低聚物 (OPV) 光学性质的影响,16 种 1,4-二苯乙烯基苯衍生物( 1a-i 和 2a-g )具有不同的给电子(ED)功能。 ) 和吸电子 (EW) 基团通过 Mizoroki-Heck 交叉偶联反应以中等至良好的产率(40-95%)合成。所实施的方法,经过我们小组先前报道的小改动,可以获得所需的乙烯基构型以及一种新型 OPV 化合物 (1h)。在通过几种技术(例如 FTIR、 1 H、 13 C 和固态核磁共振)进行结构表征后,特别强调通过紫外-可见光和荧光光谱研究它们的光学特性。结果表明,除了一个例外,OPV 系统末端的 ED 和 EW 组导致红移。随着在中心环上引入甲氧基,这种效果得到加强。与这些一致,HOMO-LUMO 间隙(ΔE)随着 ED 和 EW 取代基强度的增加而减小。ED 和 EW 取代基也会导致 Φ f 值降低。这种在
表征谱图
-
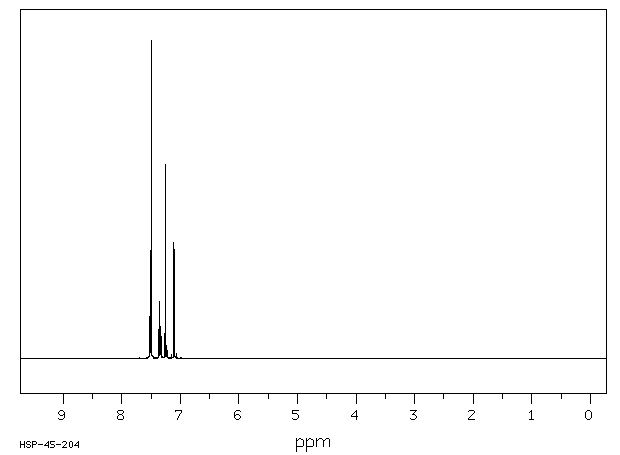
氢谱1HNMR
-
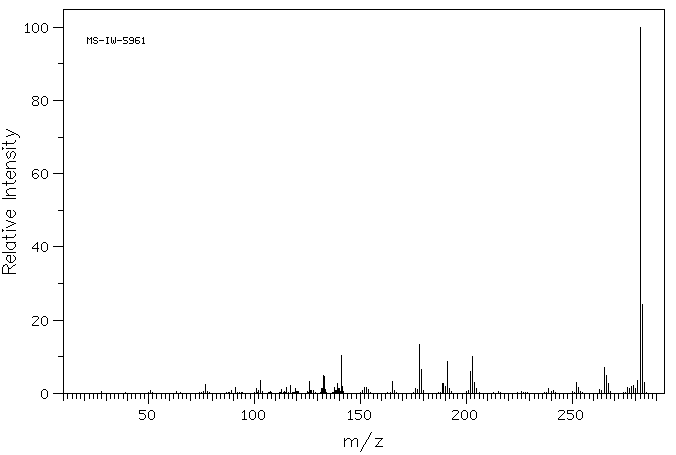
质谱MS
-
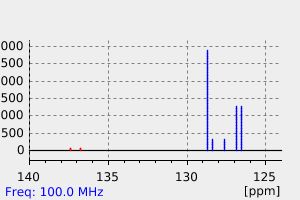
碳谱13CNMR
-
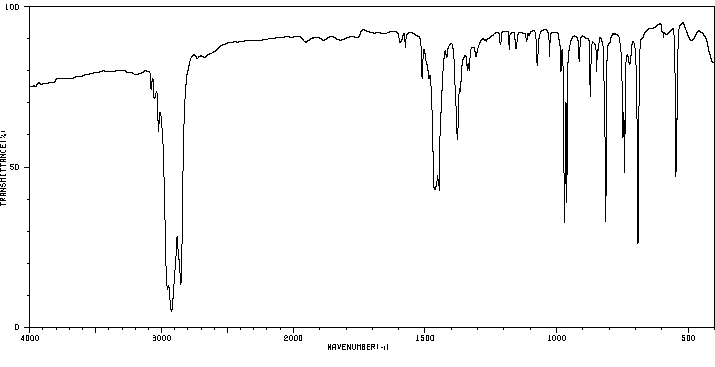
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(E,Z)-他莫昔芬N-β-D-葡糖醛酸
(E/Z)-他莫昔芬-d5
(4S,5R)-4,5-二苯基-1,2,3-恶噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S,5R,5''R)-2,2''-(1-甲基亚乙基)双[4,5-二氢-4,5-二苯基恶唑]
(4R,5S)-4,5-二苯基-1,2,3-恶噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4R,4''R,5S,5''S)-2,2''-(1-甲基亚乙基)双[4,5-二氢-4,5-二苯基恶唑]
(1R,2R)-2-(二苯基膦基)-1,2-二苯基乙胺
鼓槌石斛素
黄子囊素
高黄绿酸
顺式白藜芦醇三甲醚
顺式白藜芦醇
顺式己烯雌酚
顺式-白藜芦醇3-O-beta-D-葡糖苷酸
顺式-桑皮苷A
顺式-曲札芪苷
顺式-二苯乙烯
顺式-beta-羟基他莫昔芬
顺式-a-羟基他莫昔芬
顺式-3,4',5-三甲氧基-3'-羟基二苯乙烯
顺式-1-(3-甲基-2-萘基)-2-(2-萘基)乙烯
顺式-1,2-双(三甲基硅氧基)-1,2-双(4-溴苯基)环丙烷
顺式-1,2-二苯基环丁烷
顺-均二苯乙烯硼酸二乙醇胺酯
顺-4-硝基二苯乙烯
顺-1-异丙基-2,3-二苯基氮丙啶
非洲李(PRUNUSAFRICANA)树皮提取物
阿非昔芬
阿里可拉唑
阿那曲唑二聚体
阿托伐他汀环氧四氢呋喃
阿托伐他汀环氧乙烷杂质
阿托伐他汀环(氟苯基)钠盐杂质
阿托伐他汀环(氟苯基)烯丙基酯
阿托伐他汀杂质D
阿托伐他汀杂质94
阿托伐他汀杂质7
阿托伐他汀杂质5
阿托伐他汀内酰胺钠盐杂质
阿托伐他汀中间体M4
阿奈库碘铵
锌(II)(苯甲醛)(四苯基卟啉)
银松素
铜酸盐(5-),[m-[2-[2-[1-[4-[2-[4-[[4-[[4-[2-[4-[4-[2-[2-(羧基-kO)苯基]二氮烯基-kN1]-4,5-二氢-3-甲基-5-(羰基-kO)-1H-吡唑-1-基]-2-硫代苯基]乙烯基]-3-硫代苯基]氨基]-6-(苯基氨基)-1,3,5-三嗪-2-基]氨基]-2-硫代苯基]乙烯基]-3-硫代
铒(III) 离子载体 I
铀,二(二苯基甲酮)四碘-
钾钠2,2'-[(E)-1,2-乙烯二基]二[5-({4-苯胺基-6-[(2-羟基乙基)氨基]-1,3,5-三嗪-2-基}氨基)苯磺酸酯](1:1:1)
钠{4-[氧代(苯基)乙酰基]苯基}甲烷磺酸酯
钠;[2-甲氧基-5-[2-(3,4,5-三甲氧基苯基)乙基]苯基]硫酸盐
钠4-氨基二苯乙烯-2-磺酸酯