4-氯苄基三苯基氯化磷蓊盐 | 1530-39-8
中文名称
4-氯苄基三苯基氯化磷蓊盐
中文别名
4-(氯苄基)三苯基氯化磷鎓盐;(4-氯苄基)三苯基氯化磷;4-氯苄基三苯基氯化磷鎓盐
英文名称
(4-chlorobenzyl)triphenylphosphonium chloride
英文别名
p-chlorobenzyltriphenylphosphonium chloride;Triphenyl-(4-chlor-benzyl)-phosphoniumchlorid;[(4-chlorophenyl)methyl]triphenylphosphonium chloride;(4-chlorophenyl)methyl-triphenylphosphanium;chloride
CAS
1530-39-8
化学式
C25H21ClP*Cl
mdl
——
分子量
423.322
InChiKey
RAHOAHBOOHXRDY-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:>300 °C(lit.)
-
溶解度:在甲醇中几乎透明
-
稳定性/保质期:
如果遵照规格使用和储存,则不会分解,未有已知危险反应。应避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):2.84
-
重原子数:28
-
可旋转键数:5
-
环数:4.0
-
sp3杂化的碳原子比例:0.04
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2931900090
-
危险性防范说明:P264,P280,P302+P352+P332+P313+P362+P364,P305+P351+P338+P337+P313
-
危险性描述:H315,H319
-
储存条件:请将贮藏器保持密封,并存放在阴凉、干燥的地方。确保工作环境有良好的通风或排气设施。
SDS
| Name: | (4-Chlorobenzyl)triphenylphosphonium chloride 98% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 1530-39-8 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1530-39-8 | (4-Chlorobenzyl)triphenylphosphonium c | 98 | 216-228-0 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Causes respiratory tract irritation.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1530-39-8: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystalline powder
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 280 - 283 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: soluble in water
Specific Gravity/Density:
Molecular Formula:
Molecular Weight: 423.32
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, oxides of phosphorus, carbon dioxide.
Hazardous Polymerization: Will not occur.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1530-39-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
(4-Chlorobenzyl)triphenylphosphonium chloride - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 1530-39-8: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1530-39-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1530-39-8 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:4-氯苄基三苯基氯化磷蓊盐 在 potassium permanganate 、 正丁基锂 、 magnesium sulfate 作用下, 以 甲苯 为溶剂, 反应 53.25h, 生成 1-(4-氯苯基)-2-(4-甲苯基)乙烷-1,2-二酮参考文献:名称:Aitken, R. Alan; Cadogan, J. I. G.; Gosney, Ian, Phosphorus, Sulfur and Silicon and the Related Elements, 1995, vol. 101, # 1-4, p. 281 - 286摘要:DOI:
-
作为产物:描述:参考文献:名称:Photolysis of (arylmethyl)triphenylphosphonium salts. Substituent, counterion, and solvent effects on reaction products摘要:Quaternary (arylmethyl)phosphonium salts of the general formula ArCH2-PR3+Y-(Ar = substituted phenyl or 1-naphthyl; R = phenyl, ferrocenyl, or butyl; Y- = BF4- or halide) have been photolyzed in acetonitrile or in methanol. Photolysis involved the cleavage of the P-CH2 bond and the products derived from both, the arylmethyl radical and the carbocation, were formed. The proportion of the radical- and carbocation-derived products was determined as a function of substituents in group Ar, of groups R, counterions Y-, and the solvent. For the nonoxidizable counterion (BF4-), the proposed mechanism of the reaction involves initial homolysis, followed by the escape of the radical products from a solvent cage, or by the electron transfer from carbon to phosphorus, yielding the corresponding arylmethyl carbocation. The latter can either react with the solvent to form the observed carbocation-derived product or can undergo recombination with the tertiary phosphine formed to yield the starting phosphonium ion. Some indication of the ''inverted substituent effect'' resulting from the inhibition of single electron transfer from an easily oxidized radical was obtained. For the oxidizable counterions (halides), an additional pathway is suggested, that involves electron transfer from the anion, yielding the arylmethyl radical and the phosphine, thus decreasing the ionic/radical products ratio.DOI:10.1021/jo00073a023
-
作为试剂:参考文献:名称:Carboxyalkanoyl and hydroxycarbamoylalkanoyl derivatives of substituted摘要:化合物、组合物及使用化合物的方法缓解高血压的方法,其中化合物的结构式为##STR1##其中R为氢或较低的烷基;R.sub.1为氢、较低的烷基或苯基-较低的烷基;R.sub.2为氢、较低的烷基或苯基-较低的烷基或卤代较低的烷基;R.sub.3为羟基、--NHOH或较低的烷氧基;Pr-COOR为以下结构的取代脯氨酸##STR2##R.sub.4为卤素、酮基、偶氮基、环烷基、苯基、取代苯基、苯基-较低的烷基、取代苯基-较低的烷基、##STR3##或Y--R.sub.6;R.sub.5为氢或较低的烷基;Y为氧或硫;R.sub.6为较低的烷基、苯基、取代苯基、苯基-较低的烷基、取代苯基-较低的烷基、1-或2-萘基、取代1-或2-萘基、联苯基或取代联苯基;R.sub.7为卤素或--Y--R.sub.8;R.sub.8为较低的烷基、苯基、苯基-较低的烷基、取代苯基-较低的烷基、联苯基、萘基或R.sub.8基团连接以形成未取代的5-或6-成员环或该环中一个或多个碳原子被较低的烷基或二(较低的烷基)基取代;R.sub.9为酮基、苯基、2-或4-羟基苯基;n为0或1;以及其盐。公开号:US04311705A1
文献信息
-
Syntheses of p-terphenyls and 11,12-dihydroindeno[2,1-a]fluorene by one-pot benzannulation of Diels–Alder reactions of trans-1,2-dichloroethene and dienes作者:Jinn-Hsuan Ho、Yu-Chen Lin、Li-Ting Chou、Ying-Zhe Chen、Wei-Qi Liu、Chao-Li ChuangDOI:10.1016/j.tetlet.2013.02.002日期:2013.4p-terphenyls and 11,12-dihydroindeno[2,1-a]fluorene were successfully synthesized by one-pot benzannulation of Diels–Alder reaction with 1,2-dichloroethene as an acetylene equivalent dienophile. Two chlorine atoms could be good leaving groups to easily undergo subsequent elimination reactions of Diels–Alder products at a high temperature.
-
Postsynthetic Modification of Half-Sandwich Ruthenium Complexes by Mechanochemical Synthesis作者:Wei-Guo Jia、Xue-Ting Zhi、Xiao-Dong Li、Jun-Peng Zhou、Rui Zhong、Haibo Yu、Richmond LeeDOI:10.1021/acs.inorgchem.1c00059日期:2021.4.5A mild and environmentally friendly method to synthesize half-sandwich ruthenium complexes through the Wittig reaction between an aldehyde-tagged half-sandwich ruthenium complex and phosphorus ylide mechanochemically is reported herein. The mechanochemical synthesis of valuable half-sandwich ruthenium complexes resulted in a fast reaction, good yield with simple workup, and the avoidance of harsh reaction
-
Brønsted Acid-Catalyzed Intramolecular Hydroarylation of β-Benzylstyrenes作者:Xiao Cai、Amir Keshavarz、Justin D. Omaque、Benjamin J. StokesDOI:10.1021/acs.orglett.7b00958日期:2017.5.19triphenylmethylium tetrakis(pentafluorophenyl)borate as a convenient Brønsted acid precatalyst, β-(α,α-dimethylbenzyl)styrenes are shown to cyclize efficiently to afford a variety of new indanes that possess a benzylic quaternary center. The geminal dimethyl-containing quaternary center is proposed to be necessary to arm the substrate for cyclization through steric biasing.
-
[EN] DISUBTITUTED AZETIDINES, PYRROLIDINES, PIPERIDINES AND AZEPANES AS INHIBITORS OF MONOAMINE OXIDASE B FOR THE TREATMENT OF NEURODEGENERATIVE DISEASES<br/>[FR] AZÉTIDINES, PYRROLIDINES, PIPÉRIDINES ET AZÉPANES DI-SUBSTITUÉS EN TANT QU'INHIBITEURS DE MONOAMINE OXYDASE B POUR LE TRAITEMENT DE MALADIES NEURODÉGÉNÉRATIVES申请人:UNIV OF LJUBLJANA公开号:WO2018055096A1公开(公告)日:2018-03-29This invention relates to new inhibitors of MAO-Bwith the general formula I, where substituents are described in patent description. Compounds can be in the form of pure enantiomers or as racemic mixtures, or in the form of pharmaceutically acceptable salts. The present invention relates to the use of these inhibitors for the alleviation of symptoms and treatment of acute and chronic neurological disorders, cognitive and neurodegenerative diseases.
-
One-Pot Synthesis of Functionalized Fused Furans via a BODIPY-Catalyzed Domino Photooxygenation作者:Audrey Mauger、Jonathan Farjon、Pierrick Nun、Vincent CoeffardDOI:10.1002/chem.201706087日期:2018.4.3Six‐membered ring fused furans containing a tetrasubstituted tertiary carbon were prepared in an unprecedented one‐pot BODIPY‐catalyzed domino photooxygenation/reduction process. A series of functionalized furans was synthesized from readily available 2‐alkenylphenols and mechanistic studies were performed to account for the domino photosensitized oxygenation.
表征谱图
-
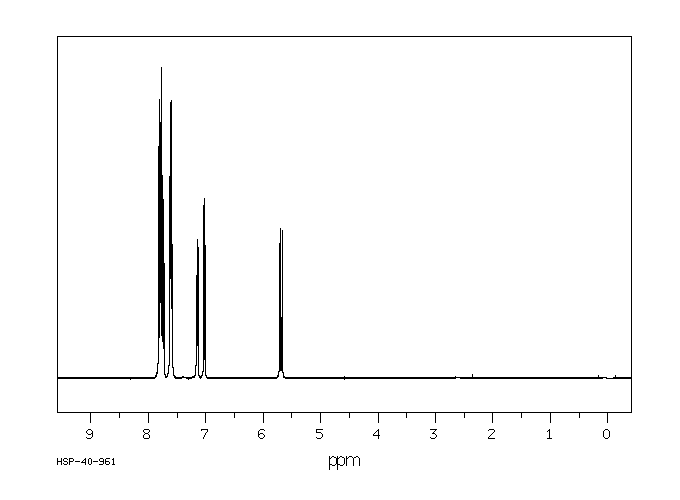
氢谱1HNMR
-
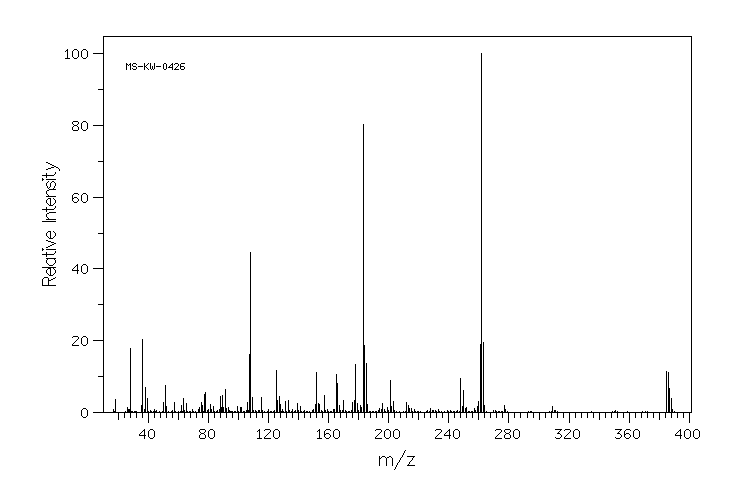
质谱MS
-
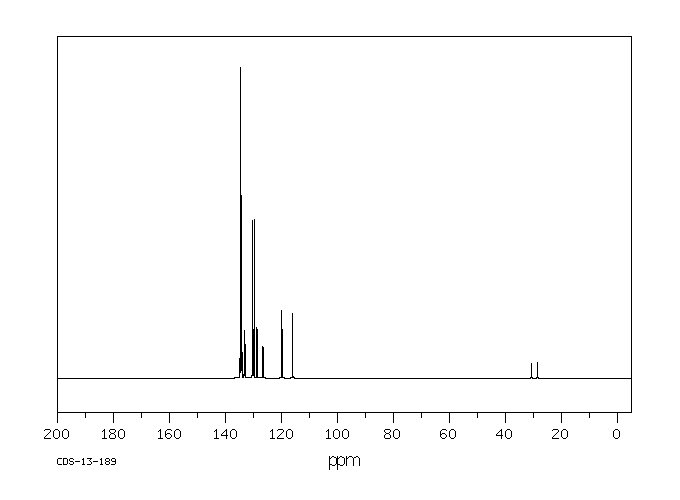
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫