1,3,4-三甲基-3-环己烯-1-羧醛 | 40702-26-9
中文名称
1,3,4-三甲基-3-环己烯-1-羧醛
中文别名
1,2,4-三甲基-4-甲酰基-1-环己烯2,6,6-三甲基环己-1-烯-1-醛
英文名称
1,3,4-trimethyl-3-cyclohexene-1-carboxaldehyde
英文别名
1,3,4-trimethyl-3-cyclohexenyl-1-carboxaldehyde;1,3,4-trimethylcyclohex-3-ene-1-carboxaldehyde;1,3,4-trimethyl-3-cyclohexene-1-carbaldehyde;1,3,4-trimethylcyclohex-3-ene-1-carbaldehyde;1,3,4-trimethyl-cyclohex-3-enecarbaldehyde;1,3,4-Trimethyl-3-cyclohexen-1-carboxaldehyde
CAS
40702-26-9
化学式
C10H16O
mdl
MFCD00060824
分子量
152.236
InChiKey
HPPUQZZCHCEJEW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:64-67 °C(Press: 5 Torr)
-
密度:0.9325 g/cm3(Temp: 420 °C)
-
LogP:2.758 (est)
-
保留指数:1171;1136
-
稳定性/保质期:
存在于主流烟气中。
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.7
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2912299000
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 1,3,4-Trimethyl-3-cyclohexen-1-carboxaldehyde
Synonyms: 1,3,4-Trimethylcyclohex-3-enecarbaldehyde
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 1,3,4-Trimethyl-3-cyclohexen-1-carboxaldehyde
CAS number: 40702-26-9
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C10H16O
Molecular weight: 152.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 1,3,4-Trimethyl-3-cyclohexen-1-carboxaldehyde
Synonyms: 1,3,4-Trimethylcyclohex-3-enecarbaldehyde
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 1,3,4-Trimethyl-3-cyclohexen-1-carboxaldehyde
CAS number: 40702-26-9
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C10H16O
Molecular weight: 152.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-(1,3,4-trimethylcyclohex-3-en-1-yl)ethanone 69461-64-9 C11H18O 166.263
反应信息
-
作为反应物:参考文献:名称:顺序狄尔斯-阿德耳反应/重排序列:官能化的双环[2.2.1]庚烷衍生物的合成及其相对构型的修订摘要:开发了一个顺序的Diels-Alder反应/重排序列,用于合成各种功能化的双环[2.2.1]庚烷,作为新型花香和木质香气。重排的结果取决于二烯的取代模式。二维NMR分析确定了最初错配的双环[2.2.1]庚酮的正确相对构型。此外,当通过手性路易斯酸催化起始的DA反应时,可以以高达96.5:3.5的对映体比例(er)获得包括(+)-丁香酮在内的双环[2.2.1]庚烷衍生物。DOI:10.1021/jo500942a
-
作为产物:描述:2-甲基丙烯醛 、 2,3-二甲基-1,3-丁二烯 在 (R)-3-(2-hydroxy-3-phenylphenyl)-2,2'-dihydroxy-1,1'-binaphthyl*3,5-bis(trifluoromethyl)benzeneboronic acid 作用下, 以 二氯甲烷 为溶剂, 反应 1.5h, 以81%的产率得到1,3,4-三甲基-3-环己烯-1-羧醛参考文献:名称:用于高对映选择性 Diels-Alder 反应的 Brønsted 酸辅助手性路易斯酸 (BLA) 催化剂的设计摘要:布朗斯台德酸辅助的手性路易斯酸 (BLA) 作为手性催化剂非常有效,可用于 α-取代和 α-未取代的 α,β-烯醛与各种二烯的对映选择性 Diels-Alder 反应。光学活性联萘酚衍生物中的羟基和具有吸电子取代基的硼试剂分别用作布朗斯台德酸和路易斯酸。手性 BLA 催化剂中的分子内布朗斯台德酸在加速 Diels-Alder 反应速率和产生高水平的对映选择性方面发挥了重要作用。特别是,由于手性 BLA 催化剂中羟基芳基在过渡态组装中的分子内氢键相互作用和有吸引力的 π-π 供体-受体相互作用,实现了优异的对映选择性。DOI:10.1021/ja9810282
文献信息
-
Carbocations as Lewis Acid Catalysts in Diels-Alder and Michael Addition Reactions作者:Juho Bah、Johan FranzénDOI:10.1002/chem.201304160日期:2014.1.20the carbocation as a highly powerful Lewis acid catalyst for organic reactions. The stable and easily available triphenylmethyl (trityl) cation was found to be a highly efficient catalyst for the Diels–Alder reaction for a range of substrates. Catalyst loadings as low as 500 ppm, excellent yields, and good endo/exo selectivities were achieved. Furthermore, by changing the electronic properties of the
-
Homogeneous catalysis. transition metal based lewis acid catalysts.作者:T. Keith Hollis、William Odenkirk、N.P. Robinson、John Whelan、B. BosnichDOI:10.1016/s0040-4020(01)87259-8日期:1993.1Transition metal based Lewis acids provide catalysts for the Diels-Alder and Mukaiyama reactions. These catalysts must possess an electron deficient axophilic metal center and a labile coordination position. Unlike traditional Lewis acids, those derived from transition metals can function in the presence of water and have well defined structures. It is shown how a normally electron rich ruthenium atom基于过渡金属的路易斯酸为Diels-Alder和Mukaiyama反应提供了催化剂。这些催化剂必须具有缺电子的亲金属中心和不稳定的配位位置。与传统的路易斯酸不同,衍生自过渡金属的那些可以在水的存在下起作用,并具有明确的结构。显示了如何通过引入吸电子配体和具有硬供体原子的配体将通常富电子的钌原子转化为路易斯酸。该钌络合物[Ru(salen)(NO)(H 2 O)] +是狄尔斯-阿尔德反应的有效催化剂,但在Mukaiyama反应中,它倾向于被还原并因此被甲硅烷基烯醇醚失活。结果表明,复数[TiCp * 2即使存在水,(H 2 O)2 ] 2+(Cp *是五甲基环戊二烯基)也是用于狄尔斯-阿尔德反应的有效催化剂。类似地,三氟配合物[TiCp 2(CF 3 SO 3)2 ]和[ZrCp 2(CF 3 SO 3)2 ](Cp为环戊二烯基)是狄尔斯-阿尔德反应和Mukaiyamayama反应的有效催化剂。所有这些催化剂在≈1
-
New Lewis acid catalysts for the Diels-Alder reaction
-
Unsaturated Aldehydes as Alkene Equivalents in the Diels–Alder Reaction作者:Esben Taarning、Robert MadsenDOI:10.1002/chem.200800003日期:2008.6.20A one-pot procedure is described for using alpha,beta-unsaturated aldehydes as olefin equivalents in the Diels-Alder reaction. The method combines the normal electron demand cycloaddition with aldehyde dienophiles and the rhodium-catalyzed decarbonylation of aldehydes to afford cyclohexenes with no electron-withdrawing substituents. In this way, the aldehyde group serves as a traceless control element描述了一种一锅法,该方法用于在Diels-Alder反应中使用α,β-不饱和醛作为烯烃当量。该方法将正常的电子需求环加成反应与醛二烯亲和剂和铑催化的醛脱羰反应结合在一起,得到不具有吸电子取代基的环己烯。以这种方式,醛基用作指导环加成反应的无痕控制元素。在催化量的三氟化硼醚化物的存在下,在二甘醇二甲醚溶液中进行Diels-Alder反应。随后路易斯酸的淬灭,添加0.3%的[Rh(dppp)2 Cl]并加热至回流实现了随后的脱羰作用,得到了环己烯产物。在这些条件下,丙烯醛,巴豆醛和肉桂醛已与多种1,3-二烯可提供环己烯的总收率在53%到88%之间。在这些转化中,这三种醛分别相当于乙烯,丙烯和苯乙烯。
-
Iminium ion catalysis: Use of the α-effect in the acceleration of the Diels–Alder reactionElectronic supplementary information (ESI) available: 1H NMR, 13C NMR, IR and MS spectra. See http://www.rsc.org/suppdata/cc/b2/b212239a/作者:Julie L. Cavill、Jens-Uwe Peters、Nicholas C. O. TomkinsonDOI:10.1039/b212239a日期:2003.3.6The alpha-effect can be used in the acceleration of the Diels-Alder reaction between a series of dienes and electron deficient dienophiles using iminium ion catalysis, providing a novel molecular scaffold capable of performing this class of catalytic process.使用亚胺离子催化,α效应可用于加速一系列二烯和电子缺陷的亲二烯体之间的Diels-Alder反应,从而提供了一种能够执行此类催化过程的新型分子支架。
表征谱图
-
氢谱1HNMR
-
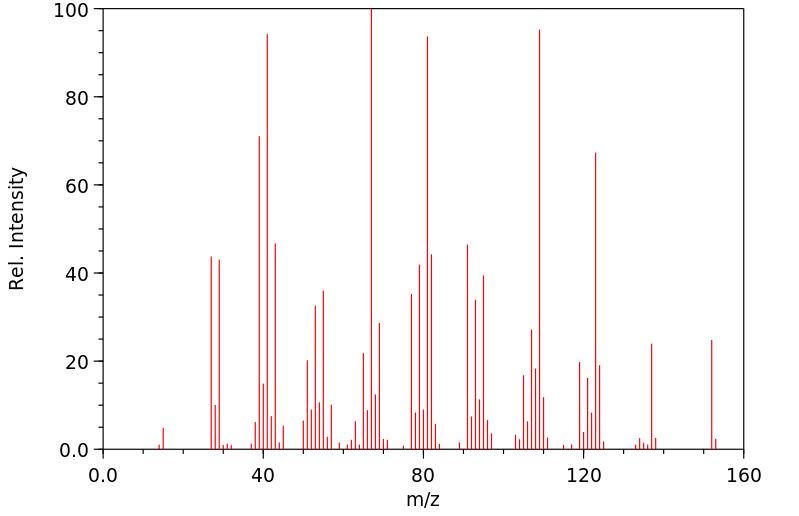
质谱MS
-
碳谱13CNMR
-
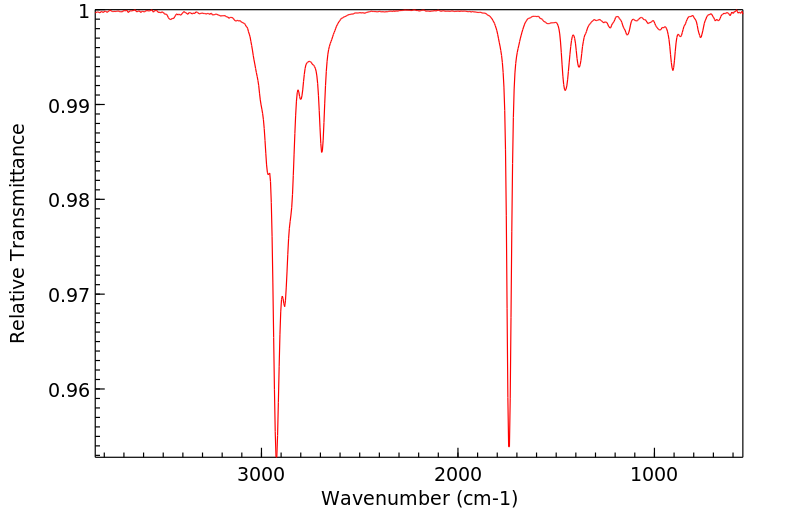
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
鲜草醛
马赛醛
顺式-环丙基-1,2-二甲醛
顺式-1-甲酰基-2-(1-己炔基)环丙烷
顺式-1,3-环己烷二甲醛
镁2-甲基丙酸盐
锶二(2-乙基-1-己醇)
锡烷,二丁基二乙氧基-
铝二异丙氧基单仲丁氧醇金属
铈(4+)四(2-甲基-2-丙醇)
過氧化二乙烷
过氧化[(1-甲基亚乙基)二-4-环己基-1-亚基]四(1,1-二甲乙基)
过氧化,3-溴丙基1,1-二甲基乙基
过氧化,1,1-二甲基乙基1-甲基乙基
表水蓼二醛
螺[4.5]癸烷-10-甲醛
聚(1-癸烯:二氧化硫)
羟基甲基叔-丁基过氧化物
甲醛-14C
甲醛-13C,D2
甲醛-13C
甲醛 [3H]
甲烷水合物
甲基6,7-二氧杂-2,3-二氮杂双环[3.2.2]壬-3,8-二烯-2-羧酸酯
甘油三酸酯过氧化物
环辛烷甲醛
环辛烷-1,4-二甲醛
环戊基甲醛
环戊基叔丁基过氧化物
环戊-3-烯-1-甲醛
环戊-2-烯-1-甲醛
环戊-2,4-二烯-1-甲醛
环戊-1,4-二烯-1-甲醛
环庚烷甲醛
环己甲醛,1,4,4-三甲基-
环己烷基甲醛
环己烷-1,3-二甲醛
环十二烷甲醛
环亚己基二[(1,1-二甲基丙基)]过氧化物
环丙甲醛
环丙-2-烯-1-甲醛
环丁基甲醛
环丁-2-烯-1-甲醛
水蓼二醛
氯代钒酸二乙酯
松油
异绒白乳菇醛
异丙醇镨(III)
异丙醇镧
异丙醇锶