noscapine | 10421-76-8
中文名称
——
中文别名
——
英文名称
noscapine
英文别名
Narcotin;Gnoskopin;Gnoscopine;6,7-dimethoxy-3-(4-methoxy-6-methyl-7,8-dihydro-5H-[1,3]dioxolo[4,5-g]isoquinolin-5-yl)-3H-2-benzofuran-1-one
CAS
10421-76-8
化学式
C22H23NO7
mdl
MFCD00127721
分子量
413.427
InChiKey
AKNNEGZIBPJZJG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
物理描述:Solid
-
熔点:230-233°C
-
溶解度:0.044 mg/mL at 20 °C
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:30
-
可旋转键数:4
-
环数:5.0
-
sp3杂化的碳原子比例:0.41
-
拓扑面积:75.7
-
氢给体数:0
-
氢受体数:8
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— Narcotolin 31210-74-9 C21H21NO7 399.4 —— narcotine N-oxide 54383-36-7 C22H23NO8 429.427 (-)-alpha-那可丁二醇 (-)-α-narcotinediol 23942-99-6 C22H27NO7 417.459 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— narcotine N-oxide 880353-82-2 C22H23NO8 429.427 —— narcotine N-oxide 54383-36-7 C22H23NO8 429.427 —— α-Anhydronarcotinediol 87633-29-2 C22H25NO6 399.444 —— 5,9-bis-(4,5-dimethoxy-3-oxo-phthalan-1-yl)-4-methoxy-6-methyl-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinoline —— C32H31NO11 605.598 —— C-(4-methoxy-6-methyl-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinolin-5-yl)-C,C'-(3,4-dimethoxy-o-phenylene)-bis-methanol 106424-06-0 C22H27NO7 417.459 那碎因 Narcein 131-28-2 C23H27NO8 445.469 —— 2,3-dimethoxy-6-{[4-methoxy-6-(2-methylamino-ethyl)-benzo[1,3]dioxol-5-yl]-acetyl}-benzoic acid 483-89-6 C22H25NO8 431.442 1,3-二氧杂环戊烯并[4,5-g]异喹啉-5-醇,5,6,7,8-四氢-4-甲氧基-6-甲基- 4-methoxy-6-methyl-5,6,7,8-tetrahydro-1,3-dioxolo[4,5-g]isoquinolin-5-ol 82-54-2 C12H15NO4 237.255
反应信息
-
作为反应物:描述:参考文献:名称:Anderson, Justus Liebigs Annalen der Chemie, 1853, vol. 86, p. 196摘要:DOI:
-
作为产物:描述:参考文献:名称:KMETTY, GEJZA;VALASIK, TIBOR;MORVIC, JAN摘要:DOI:
文献信息
-
[EN] COMPOSITIONS AND METHODS FOR MAKING NOSCAPINE AND SYNTHESIS INTERMEDIATES THEREOF<br/>[FR] COMPOSITIONS ET PROCÉDÉS DE FABRICATION DE NOSCAPINE ET D'INTERMÉDIAIRES DE SYNTHÈSE DE CELLE-CI申请人:EPIMERON INC公开号:WO2015021561A1公开(公告)日:2015-02-19Methods for the manufacture of the therapeutic chemical compound noscapine and noscapine synthesis intermediates comprising contacting a noscapine pathway precursor selected from a first canadine derivative, a first papaveroxine derivative and narcotine hemiacetal with at least one of the enzymes selected from the group CYP82Y1, CYP82X1, AT1, CYP82X2, OMT, CXE1 and NOS.
-
9-AMINONOSCAPINE AND ITS USE IN TREATING CANCERS, INCLUDING DRUG-RESISTANT CANCERS申请人:Joshi Harish C.公开号:US20110294844A1公开(公告)日:2011-12-019-aminonoscapine, prodrugs thereof, and pharmaceutically acceptable salts thereof, are disclosed. Pharmaceutical compositions including 9-aminonoscapine, and methods of preparation and use thereof are disclosed. 9-aminonoscapine is a noscapine analog that can be used to treat and/or prevent a wide variety of cancers, including drug resistant cancers, by binding tubulin and inducing apoptosis selectively in tumor cells (ovarian and T-cell lymphoma) resistant to paclitaxel, vinblastine and teniposide. 9-aminonoscapine can perturb the progression of cell cycle by mitotic arrest, followed by apoptotic cell death associated with increased caspase-3 activation and appearance of TUNEL-positive cells. Thus, 9-aminonoscapine is a novel therapeutic agents for a variety of cancers, including ovarian and T-cell lymphoma cancers, even those that have become drug-resistant to currently available chemotherapeutic drugs.
-
Synthesis and characterization of novel 1,3-benzodioxole tagged noscapine based ionic liquids with in silico and in vitro cytotoxicity analysis on HeLa cells作者:Hitesh Sehrawat、Neeraj Kumar、Ravi Tomar、Loveneesh Kumar、Vartika Tomar、Jitender Madan、Sujata K. Dass、Ramesh ChandraDOI:10.1016/j.molliq.2020.112525日期:2020.3Ionic liquids (ILs) have proven themselves as a new class of anticancer compounds among the scientific community in the 21st century. With proven efficiency of ionic liquids here an attempt has been made on a legacy anticancer compound noscapine. In this study, a library of novel noscapine (Nos) based ionic liquids were synthesized and characterized using various techniques such as 1H, 13C NMR spectroscopy离子液体(ILs)在21世纪的科学界中已证明自己是一类新型的抗癌化合物。由于离子液体已被证明具有效率,因此人们尝试了一种传统的抗癌化合物Noscapine。在这项研究中,使用诸如1 H,13 C NMR光谱和质谱等各种技术合成并鉴定了基于新型Noscapine(Nos)的离子液体库。通过计算机研究了这些新型的基于Nos的离子液体包括分子对接分析在内的实验表明,基于Nos的离子液体的[Pip-Nos] OAc和[Pip-Nos] OTf衍生物具有较高的分子结合力,对接分数分别为-336.19 kJ / mol和-326.71 kJ / mol,远远高于母体化合物Noscapine(−267.06 kJ / mol)。同样,药代动力学和药效学性质分析显示了良好的相似度,并获得了令人满意的结果。通过在HeLa癌细胞系上进行的体外抗癌细胞毒性试验进一步验证了前导化合物。在体外细胞毒性分析所描绘的铅化合物的高效力的抗癌具有较低IC
-
Boran-Addukte des Narkotins und Hydrastins und ihrer Reduktionsprodukte作者:Silvia Prior、Wolfgang WiegrebeDOI:10.1002/ardp.19833160902日期:——α‐Narkotin (3), β‐Hydrastin (6) und ihre Reduktionsprodukte binden Boran koordinativ am Stickstoff. — Aus α‐Narkotindiol (8) entsteht mit K‐tert. butylat/Benzophenon nicht α‐Anhydronarkotindiol7), sondern eine Quettamin‐ähnliche Verbindung 9 ohne Racemisierung am Benzyl‐C.
-
Biomimetic photooxidation of noscapine sensitized by a riboflavin derivative in water: The combined role of natural dyes and solar light in environmental remediation作者:Alice Pavanello、Debora Fabbri、Paola Calza、Debora Battiston、Miguel A. Miranda、M. Luisa MarinDOI:10.1016/j.jphotobiol.2022.112415日期:2022.4Noscapine (NSC) is a benzyl-isoquinoline alkaloid discovered in 1930 as an antitussive agent. Recently, NSC has also been reported to exhibit antitumor activity and, according to computational studies, it is able to attack the protease enzyme of Coronavirus (COVID-19) and thus could be used as antiviral for COVID-19 pandemic. Therefore, an increasing use of this drug could be envisaged in the coming诺斯卡品 (NSC) 是一种苄基异喹啉生物碱,于 1930 年被发现作为镇咳剂。最近,据报道,NSC 具有抗肿瘤活性,根据计算研究,它能够攻击冠状病毒 (COVID-19) 的蛋白酶,因此可用作 COVID-19 大流行的抗病毒药物。因此,可以设想在未来几年内越来越多地使用这种药物。NSC 很容易被代谢,半衰期为 4.5 小时,产生 coTArnine、hydrocoTArnine 和 meconine,由 C 的氧化破坏产生异喹啉和苯酞部分之间的 C 键。由于其潜在的使用增加,高浓度的 NSC 及其代谢物将被释放到环境中,并可能影响自然生态系统。因此,这项工作的目的是研究在天然存在的光催化剂存在下 NSC 的降解。事实上,目前的贡献已经证明,在天然有机染料核黄素 (RFTA) 的衍生物存在下,NSC 可以在暴露于可见光下有效降解。事实上,对光降解机制的详细研究揭示了仿生和光催化过程之间的相似性。事实上,基于仔细的
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
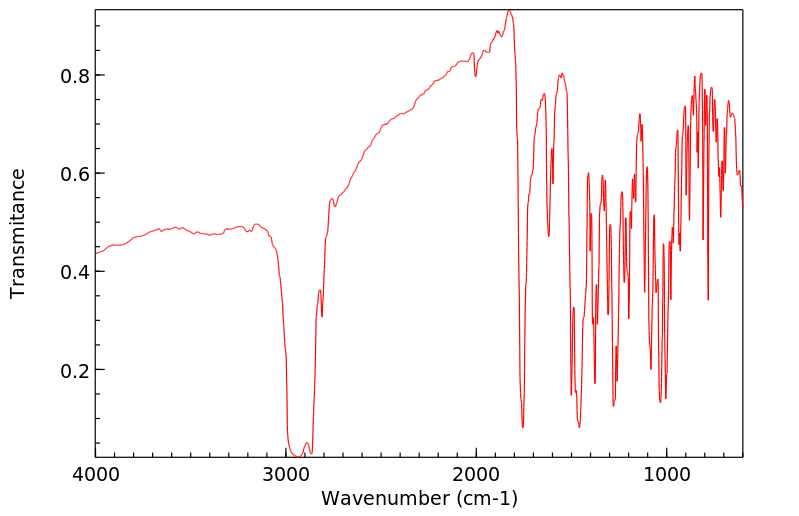
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
阿托喹啉
那可汀
那可丁N-氧化物
诺司卡品 盐酸盐 水合物
细果角茴香碱
紫堇明
盐酸那可丁一水合物
盐酸诺格考平
盐酸白毛莨碱
曲托喹啉
山缘草定碱
咖喏定
北美黄连碱
[S-(R*,R*)]-6,7-二甲氧基-3-(5,6,7,8-四氢-4-羟基-6-甲基-1,3-二氧杂环戊并[4,5-g]异喹啉-5-基)苯酞
[6S,(+)]-6-[(1S)-1,2,3,4-四氢-6,7-二甲氧基-2-甲基异喹啉-1-基]呋喃并[3,4-e]-1,3-苯并二氧戊环-8(6H)-酮
7-氨基-4,5,6-三乙氧基-3-(6,7,8-三甲氧基-2-甲基-3,4-二氢-1H-异喹啉-1-基)-3H-2-苯并呋喃-1-酮
7-O-去甲基alpha-那可丁
6,7-二甲氧基-3-[(5R)-4-甲氧基-6-甲基-7,8-二氢-5H-[1,3]二氧杂环戊并[4,5-g]异喹啉-5-基]-3H-2-苯并呋喃-1-酮
3-异喹啉-1-基-3H-2-苯并呋喃-1-酮
(3S)-6,7-二甲氧基-3-[(5S)-6-甲基-5,6,7,8-四氢[1,3]二氧杂环戊并[4,5-g]异喹啉-5-基]-2-苯并呋喃-1(3H)-酮
(3S)-3-[(1R)-6,7-二羟基-8-甲氧基-2-甲基-3,4-二氢-1H-异喹啉-1-基]-6,7-二甲氧基-3H-2-苯并呋喃-1-酮
(-)-荷苞牡丹碱甲溴化物
(-)-荷苞牡丹碱
(-)-荷包牡丹碱甲碘化物
(-)-荷包牡丹碱甲溴化物
(-)-荷包牡丹碱甲氯化物
(-)-紫堇明
(+)-荷苞牡丹碱甲氯化物
(+)-荷包牡丹碱
8-Isopentyl-narcotolin
8-<4-Chlor-benzyl>-narcotolin
(R)-5-((R)-4,5-dimethoxy-3-oxo-phthalan-1-yl)-4-methoxy-6,6-dimethyl-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinolinium; iodide
8-Allylnarkotolin
8-p-Nitrobenzylnarkotolin
2-(thiazolidin-3-yl)ethyl (E)-6-[1,3-dihydro-4-(N-(trifluoroacetyl)-N-isopropyl)amino-6-methoxy-7-methyl-3-oxoisobenzofuran-5-yl]-4-methyl-4-hexenoate
2-[5-(4,5-Dimethoxy-3-oxo-1,3-dihydro-isobenzofuran-1-yl)-6-methyl-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinolin-4-yloxy]-N-(4-ethoxy-phenyl)-acetamide
8-Narcotolinessigsaeureethylester
2-[5-(4,5-Dimethoxy-3-oxo-1,3-dihydro-isobenzofuran-1-yl)-6-methyl-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinolin-4-yloxy]-N-(4-sulfamoyl-phenyl)-acetamide
6-(6',7'-diacetoxy-2'-methyl-1',2',3',4'-tetrahydroisoquinolin-1'-yl)furo<3,4-e>-1,3-benzodioxol-8(6H)-one hydrobromide
6,7-dimethoxy-3(7-methoxy-2-methyl-1,2,3,4-tetrahydroisoquinolin-1-yl)isobenzofuran-1(3H)-one
5-((R)-4,5-Dimethoxy-3-oxo-1,3-dihydro-isobenzofuran-1-yl)-6-methyl-7,8-dihydro-[1,3]dioxolo[4,5-g]isoquinolin-6-ium
(+/-)-erythro-1-<1'-(4',5'-dimethoxyphthalidyl)>-2-methyl-1,2,3,4-tetrahydroisoquinoline
6-(6',7'-dihydroxy-2'-methyl-1',2',3',4'-tetrahydroisoquinolin-1'-yl)furo<3,4-e>-1,3-benzodioxol-8(6H)-one hydrobromide
4-{2-[5-(4,5-Dimethoxy-3-oxo-1,3-dihydro-isobenzofuran-1-yl)-6-methyl-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinolin-4-yloxy]-acetylamino}-benzoic acid ethyl ester
Corledin-acetat
(-)-α-hydrastine; hydrochloride
N-Aminonarcotiniumion
3d-β-Narcotin
rac-2,3;10,11-bis-methanediyldioxy-16ξ-methyl-16ξ-oxy-rheadan-8-one
(1R,5R)-8,9,18,19-tetramethoxy-3-oxa-13-azapentacyclo[11.8.0.01,5.06,11.016,21]henicosa-6,8,10,16,18,20-hexaene-4,12-dione