1-(5-溴-1-苯并噻吩-3-基)乙酮 | 1423-63-8
中文名称
1-(5-溴-1-苯并噻吩-3-基)乙酮
中文别名
3-乙酰基-5-溴苯并噻吩
英文名称
1-(5-bromobenzo[b]thiophen-3-yl)ethan-1-one
英文别名
1-(5-bromobenzo[b]thiophen-3-yl)ethanone;3-acetyl-5-bromobenzo[b]thiophene;1-(5-bromo-benzo[b]thiophen-3-yl)-ethanone;3-acetyl-5-bromo-benzothiophene;3-Acetyl-5-brom-benzothiophen;1-(5-bromo-1-benzothiophen-3-yl)ethanone
CAS
1423-63-8
化学式
C10H7BrOS
mdl
——
分子量
255.135
InChiKey
IRGMTUHYELTYEG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:112-113°C
-
沸点:359.7±22.0 °C(Predicted)
-
密度:1.587±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:13
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:45.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险类别码:R20/21/22
-
海关编码:2934999090
SDS
| Name: | 1-(5-Bromo-1-benzothien-3-yl)ethanone Material Safety Data Sheet |
| Synonym: | |
| CAS: | 1423-63-8 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1423-63-8 | 1-(5-Bromo-1-benzothien-3-yl)ethanone | 97+% | unlisted |
Risk Phrases: 20/21/22 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
Causes skin irritation. Harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
Inhalation:
Harmful if inhaled. Causes respiratory tract irritation.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid. Get medical aid immediately.
Skin:
Get medical aid. Get medical aid immediately. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Do not induce vomiting. Get medical aid. Get medical aid immediately. Wash mouth out with water.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use foam, dry chemical, or carbon dioxide. Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes. Do not breathe dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing. Use only in a chemical fume hood.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1423-63-8: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: Off White
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 112 - 113 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
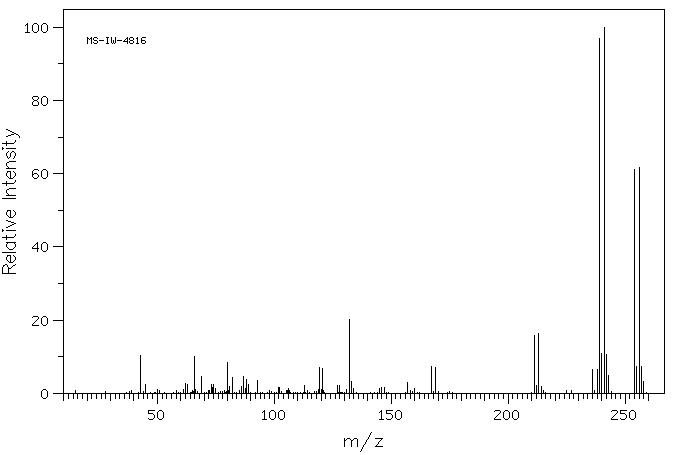
Molecular Formula: C10H7BrOS
Molecular Weight: 255.13
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents, bases, amines, halogens.
Hazardous Decomposition Products:
Carbon monoxide, oxides of sulfur, carbon dioxide, hydrogen bromide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1423-63-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1-(5-Bromo-1-benzothien-3-yl)ethanone - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.*
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
IMO
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing Group: III
RID/ADR
Shipping Name: TOXIC SOLID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2811
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 22 Do not breathe dust.
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
WGK (Water Danger/Protection)
CAS# 1423-63-8: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1423-63-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1423-63-8 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 5-溴苯并[B]噻吩-3-甲酸 5-bromobenzo[b]thiophene-3-carboxylic acid 7312-24-5 C9H5BrO2S 257.107 —— 1-(5-Pyrimidin-5-yl-1-benzothiophen-3-yl)ethanone 1049001-61-7 C14H10N2OS 254.312 5-羟基苯并[B]噻吩-3-甲酸 5-hydroxybenzo[b]thiophene-3-carboxylic acid 16304-39-5 C9H6O3S 194.211
反应信息
-
作为反应物:描述:1-(5-溴-1-苯并噻吩-3-基)乙酮 在 titanium(IV) tetraethanolate 、 copper(l) iodide 、 (1R,2R)-(-)-N,N'-二甲基-1,2-环己二胺 、 sodium carbonate 、 potassium carbonate 、 三氟乙酸 、 间苯二甲醚 作用下, 以 四氢呋喃 、 甲醇 、 二氯甲烷 、 甲苯 为溶剂, 反应 54.17h, 生成 (R)-4-fluoro-N-(3-(1-(N-methylsulfamoyl)-2-thioureidopropan-2-yl)benzo[b]thiophen-5-yl)benzamide参考文献:名称:亚氨基噻二嗪二氧化物衍生物及其用途摘要:本发明公开了亚氨基噻二嗪二氧化物衍生物及其用途,具体地,本发明涉及一类新颖的亚氨基噻二嗪二氧化物衍生物以及包含该类化合物的药物组合物,它们可作为BACE‑1抑制剂。本发明还涉及制备这类化合物和药物组合物的方法,以及它们在制备治疗与β‑淀粉样蛋白(“Aβ”)有关的疾病,特别是阿尔茨海默病的药物中的用途。公开号:CN109796447B
-
作为产物:描述:参考文献:名称:一类新型的,有效的,选择性的和口服活性的前列腺素D2受体拮抗剂的各种衍生物的合成和生物活性。2.6,6-二甲基双环[3.1.1]庚烷衍生物。摘要:在较早的论文中,我们报道了以双环[2.2.1]庚烷环系统为前列腺素骨架的新型前列腺素D(2)(PGD(2))受体拮抗剂是一种有效的新型抗过敏剂,可抑制各种过敏性炎症诸如在结膜炎和哮喘模型中观察到的反应。在本研究中,我们合成了具有6,6-二甲基双环[3.1.1]庚烷环系统的PGD(2)受体拮抗剂。与具有磺酰胺基的双环[2.2.1]庚烷环系统的那些相反,这些衍生物具有酰胺部分。具有6,6-二甲基双环[3.1.1]庚烷环的衍生物在PGD(2)受体结合和cAMP形成分析中也显示出强大的活性。在诸如过敏性鼻炎,结膜炎和哮喘模型等体内分析中,这些系列的衍生物显示出优异的药理特性。特别地,化合物45在过敏性鼻炎和哮喘模型中也有效抑制了嗜酸性粒细胞的浸润。该化合物(45,S-5751)现在正被开发为一种有前途的抗过敏药物候选物。DOI:10.1021/jm0205189
文献信息
-
Hydrogen bond donor solvents enabled metal and halogen-free Friedel–Crafts acylations with virtually no waste stream作者:Guangchang Liu、Bo XuDOI:10.1016/j.tetlet.2018.01.026日期:2018.3halogen-free Friedel–Crafts acylation protocol with virtually no waste stream generation. We propose a hydrogen bonding donor solvent will form a hydrogen bonding network and may provide significant rate enhancement for Friedel–Crafts reactions. Trifluoroacetic acid is one of the strongest H-bond donor solvents, which is also volatile and can be easily recovered by distillation without need for reaction workup
-
FUNCTIONALLY SELECTIVE ALPHA2C ADRENORECEPTOR AGONISTS申请人:De lera Ruiz Manuel公开号:US20100197562A1公开(公告)日:2010-08-05In its many embodiments, the present invention provides a novel class of biaryl compounds as inhibitors of ÿ2C adrenergic receptor agonists, methods of preparing such compounds, pharmaceutical compositions containing one or more such compounds, methods of preparing pharmaceutical formulations comprising one or more such compounds, and methods of treatment, prevention, inhibition, or amelioration of one or more conditions associated with the ÿ2C adrenergic receptors using such compounds or pharmaceutical compositions.
-
Compounds, pharmaceutical compositions, and methods for inhibiting protein kinases申请人:——公开号:US20020006952A1公开(公告)日:2002-01-17Amino-pyrazole compounds that modulate and/or inhibit the activity of protein kinases. These compounds and pharmaceutical compositions containing them are capable of mediating and/or inhibiting the activity cyclin-dependent kinases, thereby modulating and/or inhibiting unwanted cell proliferation. The invention is also directed to the therapeutic or prophylactic use of pharmaceutical compositions containing such compounds, and to methods of treating cancer as well as other disease states associated with unwanted angiogenesis and/or cellular proliferation, such as diabetic retinopathy, neovascular glaucoma, rheumatoid arthritis, and psoriasis, by administering effective amounts of such compounds.
-
Process for preparing 5-hydroxybenzo [b] thiophene-3-carboxylic acid derivatives申请人:——公开号:US20020026061A1公开(公告)日:2002-02-28Benzothiophenecarboxylic acid derivatives of the formula (I) which are useful as starting materials for producing drugs, and a process for preparing 5-hydroxybenzo[b]-thiophene-3-carboxylic acid derivatives of the formula (VI) which are specific PGD 2 antagonists. 1
-
PROCESS FOR PREPARING 5-HYDROXYBENZO [B] THIOPHENE-3-CARBOXYLIC ACID DERIVATIVES申请人:——公开号:US20020016476A1公开(公告)日:2002-02-07Benzothiophenecarboxylic acid derivatives of the formula (I) which are useful as starting materials for producing drugs, and a process for preparing 5-hydroxybenzo[b]-thiophene-3-carboxylic acid derivatives of the formula (VI) which are specific PGD 2 antagonists. 1
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
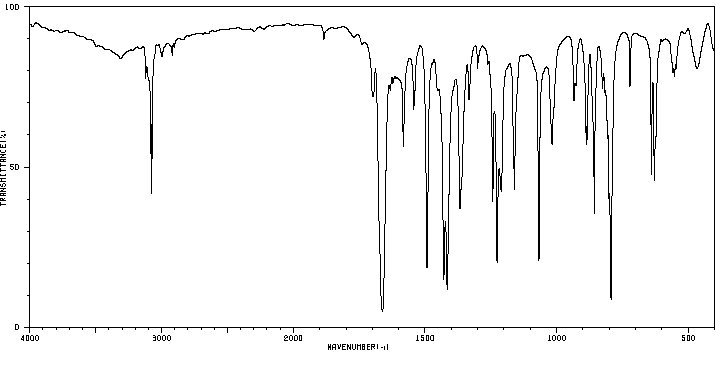
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐留通钠
齐留通相关物质A
齐留通亚砜
齐留通-d4
齐留通
雷洛昔芬杂质
邻联甲苯胺砜
试剂4,8-Bis(3,5-dioctyl-2-thienyl)-2,6-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[1,2-b:4,5-b']dithiophene
试剂1,1'-[4,8-Bis[4-(2-ethylhexyl)-3,5-difluorophenyl]benzo[1,2-b:4,5-b']dithiophene-2,6-diyl]bis[1,1,1-trimethylstannane]
苯并噻吩-7-醇
苯并噻吩-4-硼酸频哪醇酯
苯并噻吩-3-羧酸甲酯
苯并噻吩-3-硼酸
苯并噻吩-2-羰酰氯
苯并噻吩-2-羧酸肼
苯并噻吩-2-羧酸
苯并噻吩-2-硼酸
苯并噻吩-2-氨基甲酸叔丁酯
苯并噻吩
苯并[c]噻吩
苯并[b]噻吩-7-胺
苯并[b]噻吩-7-羧酸乙酯
苯并[b]噻吩-7-甲醛
苯并[b]噻吩-7-甲腈
苯并[b]噻吩-6-醇
苯并[b]噻吩-6-胺
苯并[b]噻吩-6-羧酸乙酯
苯并[b]噻吩-6-羧酸
苯并[b]噻吩-6-甲腈
苯并[b]噻吩-5-甲腈,2-甲酰基-
苯并[b]噻吩-5-甲磺酰氯
苯并[b]噻吩-4-羧酸甲酯
苯并[b]噻吩-4-羧酸
苯并[b]噻吩-4-甲醛
苯并[b]噻吩-4-甲腈
苯并[b]噻吩-4-基甲醇
苯并[b]噻吩-3-胺盐酸盐
苯并[b]噻吩-3-胺
苯并[b]噻吩-3-羧酸-(2-二烯丙基氨基乙酯)
苯并[b]噻吩-3-硼酸频哪酯
苯并[b]噻吩-3-甲醛肟
苯并[b]噻吩-3-甲酰胺
苯并[b]噻吩-3-基乙酸酯
苯并[b]噻吩-3-乙酸
苯并[b]噻吩-3-乙酰氯
苯并[b]噻吩-3-乙腈
苯并[b]噻吩-2-胺盐酸盐
苯并[b]噻吩-2-羧酸6-氨基-3-氯-甲酯
苯并[b]噻吩-2-羧酸,5-氯-3-(1-甲基乙氧基)-
苯并[b]噻吩-2-羧酸,3-羟基-5-甲氧基-,甲基酯