3-methyl-1-hexanol | 111768-08-2
中文名称
——
中文别名
——
英文名称
3-methyl-1-hexanol
英文别名
3-methyl-hexan-1-ol;racemic 3-methyl-hexyl alcohol;(+/-)-3-methyl-hexan-1-ol;racemischer 3-Methyl-hexylalkohol;γ-Methyl-n-hexylalkohol;(+/-)-3-Methylhexylalkohol (XI);3-methylhexan-1-ol
CAS
111768-08-2
化学式
C7H16O
mdl
——
分子量
116.203
InChiKey
YGZVAQICDGBHMD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-30.45°C (estimate)
-
沸点:147.3°C (estimate)
-
密度:0.8258
-
保留指数:895
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:8
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-甲基已烷 3-methyl-hexane 589-34-4 C7H16 100.204
反应信息
-
作为反应物:描述:参考文献:名称:新型基于硫噻吩并[2,3-d]嘧啶-4-基zone的细胞周期蛋白依赖性激酶4抑制剂的发现:合成,生物学评估和构效关系。摘要:描述,设计和合成新型噻吩并[2,3-d]嘧啶-4-基类似物作为细胞周期蛋白依赖性激酶4(CDK4)抑制剂。在继续我们的计划以寻找有效的CDK4抑制剂的过程中,在part部分引入噻唑基团导致化学稳定性显着提高。此外,通过集中于噻唑环的C-4'位置和噻吩并[2,3-d]嘧啶部分的C-6位置的优化,已确定化合物35在HCT116异种移植模型中有效细胞。在本文中,我们对合成化合物的效价,选择性和结构活性关系进行了讨论。DOI:10.1248/cpb.59.991
-
作为产物:描述:参考文献:名称:Weitzel et al., Hoppe-Seyler's Zeitschrift fur Physiologische Chemie, 1952, vol. 291, p. 29,40摘要:DOI:
文献信息
-
Oxidations catalyzed by osmium compounds. Part 1: Efficient alkane oxidation with peroxides catalyzed by an olefin carbonyl osmium(0) complex作者:Georgiy B. Shul’pin、Aleksandr R. Kudinov、Lidia S. Shul’pina、Elena A. PetrovskayaDOI:10.1016/j.jorganchem.2005.10.028日期:2006.24-diphenylbut-2-en-1,4-dione)undecacarbonyl triangulotriosmium (1), efficiently catalyzes oxygenation of alkanes (cyclohexane, cyclooctane, n-heptane, isooctane, etc.) with hydrogen peroxide, as well as with tert-butyl hydroperoxide and meta-chloroperoxybenzoic acid in acetonitrile solution. Alkanes are oxidized to corresponding alcohols, ketones (aldehydes) and alkyl hydroperoxides. Thus, heating cyclooctane with the与π配位烯烃(2,3-η-1,4-diphenylbut-2-en-1,4-dione)十一碳羰基三氟ang(1)形成的羰基os(0)有效催化链烷烃(环己烷,环辛烷,正庚烷,异辛烷等),过氧化氢以及叔丁基氢过氧化物和间氯过氧苯甲酸的乙腈溶液。烷烃被氧化为相应的醇,酮(醛)和烷基氢过氧化物。因此,用1 –H 2 O 2加热环辛烷在70°C下混合后,经过6小时后,产品的营业额高达2400。以环己烷计,所有产物的最大产率均等于20%,以H 2 O 2计,则为30%。直链和支链烷烃的氧化表现出非常低的区域选择性和键选择性参数,这证明该反应是通过羟基自由基攻击烷烃的CH键进行的。当在氩气氛下进行反应时,没有形成氧合产物,因此可以得出结论,氧合是通过烷基与大气氧之间的反应而发生的。总而言之,Os(0)络合物比任何可溶的铁衍生物(在周期系统中是analogue的类似物)都更强大地产生羟基自由基。
-
Alkane oxygenation with H2O2 catalysed by FeCl3 and 2,2′-bipyridine作者:Georgiy B. Shul’pin、Camilla C. Golfeto、Georg Süss-Fink、Lidia S. Shul’pina、Dalmo MandelliDOI:10.1016/j.tetlet.2005.05.007日期:2005.7acetonitrile efficiently oxidises alkanes predominantly to alkyl hydroperoxides. Turnover numbers attain 400 after 1 h at 60 °C. It has been assumed that bipy facilitates proton abstraction from a H2O2 molecule coordinated to the iron ion (these reactions are stages in the catalytic cycle generating hydroxyl radicals from the hydrogen peroxide). Hydroxyl radicals then attack alkane molecules finally yielding
-
PROCESS FOR DOUBLE CARBONYLATION OF ALLYL ALCOHOLS TO CORRESPONDING DIESTERS申请人:EVONIK DEGUSSA GMBH公开号:US20170174610A1公开(公告)日:2017-06-22The invention relates to a process for doubly carbonylating allyl alcohols to the corresponding diesters, wherein a linear or branched allyl alcohol is reacted with a linear or branched alkanol (alcohol) with supply of CO and in the presence of a catalytic system composed of a palladium complex and at least one organic phosphorus ligand and in the presence of a hydrogen halide selected from HCl, HBr and HI.
-
[EN] CARBOHYDRATE-MEDIATED TUMOR TARGETING<br/>[FR] CIBLAGE DE TUMEURS MÉDIÉ PAR UN HYDRATE DE CARBONE申请人:UNIV ARIZONA公开号:WO2011019419A1公开(公告)日:2011-02-17Tumors can be selectively targeted via compounds provided herein according to the formula, or a pharmaceutically acceptable salt thereof, wherein RA and RB are as defined herein. Tumors can be imaged or targeted for therapeutic treatment using compounds described herein where at least one RA or at least one RB group comprises a imaging agent, a therapeutic agent, or a member of a specific binding pair which can be associated with a secondary imaging agent, such as a microbubble for ultrasonic imaging.本文提供的化合物可选择性地针对肿瘤,其中根据以下公式或其药用可接受的盐,其中RA和RB如本文所定义。肿瘤可通过本文描述的化合物进行成像或靶向治疗,其中至少一个RA或至少一个RB基团包含成像剂、治疗剂或特定结合对的成员,可与次级成像剂(如用于超声成像的微泡)结合。
-
Erythropoietin Expression Promoter申请人:TOHOKU UNIVERSITY公开号:US20150353489A1公开(公告)日:2015-12-10The present invention provides an erythropoietin expression-enhancing agent that can cancel the suppression of erythropoietin production or promote erythropoietin production, and a therapeutic or preventive drug for anemia, a liver function-improving agent, an ischemic injury-improving agent, a renal protective agent, and an insulin secretagogue comprising the erythropoietin expression-enhancing agent. The erythropoietin expression-enhancing agent of the present invention comprises one or more compounds selected from the group consisting of compounds represented by the following general formulas (I), (II), and (III) and pharmaceutically acceptable salts thereof when R 3 is OH.
表征谱图
-
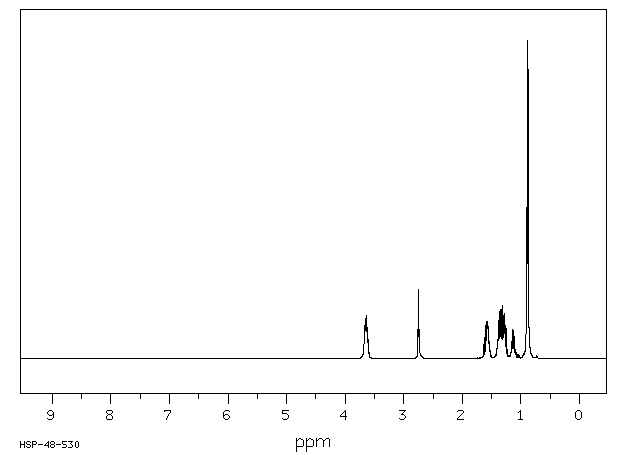
氢谱1HNMR
-
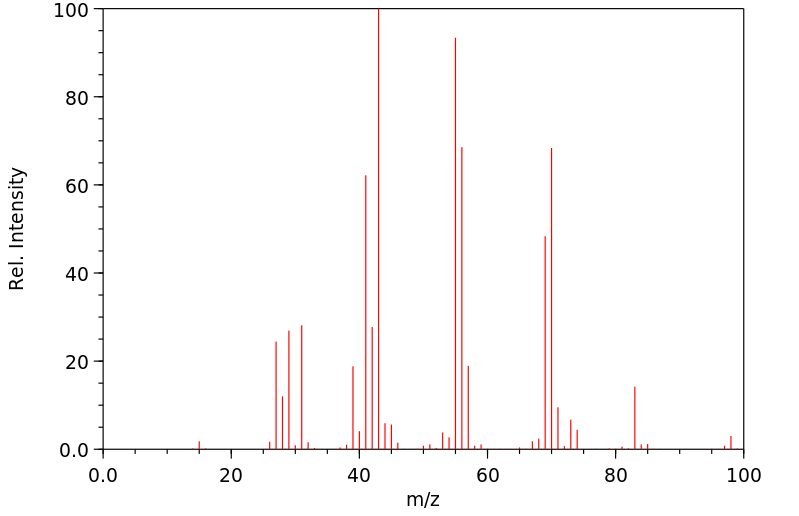
质谱MS
-
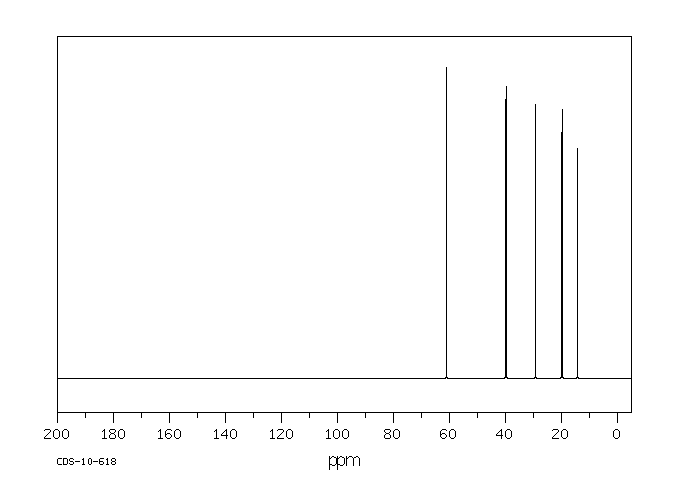
碳谱13CNMR
-
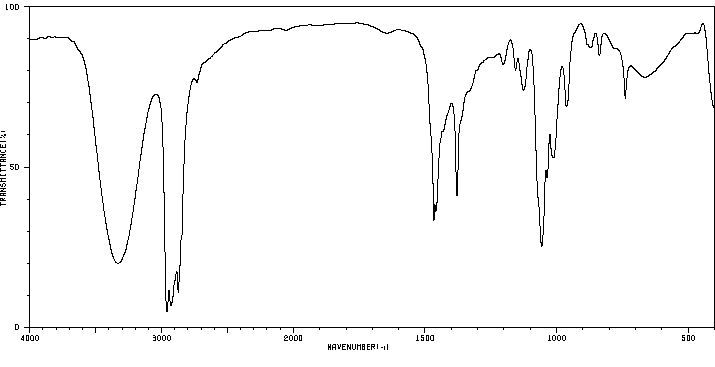
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯