4-丙氧基苯甲腈 | 60758-84-1
中文名称
4-丙氧基苯甲腈
中文别名
——
英文名称
4-propoxybenzonitrile
英文别名
——
CAS
60758-84-1
化学式
C10H11NO
mdl
MFCD00173874
分子量
161.203
InChiKey
VYOPNUZYNSNYRA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
稳定性/保质期:
避免接触氧化物
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:12
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:33
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xn
-
安全说明:S36/37
-
危险类别码:R20/21/22
-
海关编码:2926909090
-
储存条件:在密封的贮藏器中存放,并置于阴凉、干燥处。
SDS
| Name: | 4-Propoxybenzonitrile 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 60758-84-1 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 60758-84-1 | 4-Propoxybenzonitrile | 97% | unlisted |
Risk Phrases: 20/21/22
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. Harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
Inhalation:
Harmful if inhaled. May cause respiratory tract irritation.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 60758-84-1: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C10H11NO
Molecular Weight: 161
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Acids, bases, oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 60758-84-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
4-Propoxybenzonitrile - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: NITRILES, SOLID, TOXIC, N.O.S.*
Hazard Class: 6.1
UN Number: 3276
Packing Group: III
IMO
Shipping Name: NITRILES, TOXIC, N.O.S.
Hazard Class: 6.1
UN Number: 3276
Packing Group: III
RID/ADR
Shipping Name: NITRILES, TOXIC, N.O.S.
Hazard Class: 6.1
UN Number: 3276
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
Safety Phrases:
S 36/37 Wear suitable protective clothing and
gloves.
WGK (Water Danger/Protection)
CAS# 60758-84-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 60758-84-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 60758-84-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-(烯丙氧基)苯甲腈 4-allyloxy-benzonitrile 33148-47-9 C10H9NO 159.188 1-丙氧基-4-乙烯基苯 1-propoxy-4-vinylbenzene 80122-67-4 C11H14O 162.232 —— 4-propyloxybenzaldoxime 61096-85-3 C10H13NO2 179.219 4-羟基苯甲腈 4-cyanophenol 767-00-0 C7H5NO 119.123 4-苯甲氧基苯甲腈 4-benzyloxybenzonitrile 52805-36-4 C14H11NO 209.247 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (4-丙氧基苯基)甲醇 (4-propoxyphenyl)methanol 90925-43-2 C10H14O2 166.22 对丙氧基苯甲酰胺 1-(4-propoxyphenyl)methanamine 21244-33-7 C10H15NO 165.235 4-羟基苯甲腈 4-cyanophenol 767-00-0 C7H5NO 119.123
反应信息
-
作为反应物:描述:4-丙氧基苯甲腈 在 1-丁基-3-甲基咪唑溴盐 作用下, 反应 0.5h, 以96%的产率得到4-羟基苯甲腈参考文献:名称:在微波照射下使用离子液体高效裂解烷基芳基醚摘要:已经开发了一种高度可靠的烷基芳基醚脱烷基方案,其烷基比甲基长。我们报告了使用离子液体 1-n-丁基-3-甲基咪唑溴化物 [bmim][Br] 在微波照射下成功裂解了各种乙基、正丙基和苄基芳基醚。尽管烷基化剂具有成本低、毒性小、产品疏水性大等许多特点,但较长的烷基醚比甲基醚的开发要少得多,这可能是由于脱保护步骤的难度更大。由于与本组开发的去甲基化方法具有条件温和、反应时间短、离子液体用量少等优点,脱烷基协议可以极大地鼓励更广泛地使用更长的烷基来保护酚基。与我们之前使用 [bmim][Br] 进行去甲基化的研究一样,微波辐射对于较长的烷基芳基醚的脱保护至关重要。与导致低转化率或分解的传统加热不同,微波辐射似乎更有效地提供能量来裂解醚键,因此抑制了不希望的反应。DOI:10.5012/bkcs.2013.34.1.174
-
作为产物:描述:4-(烯丙氧基)苯甲腈 在 tetrabutylammonium tetrafluoroborate 、 potassium carbonate 作用下, 以 N,N-二甲基甲酰胺 、 丁酮 为溶剂, 反应 15.0h, 生成 4-丙氧基苯甲腈参考文献:名称:电化学还原烷基芳基醚获得的自由基阴离子中碳-氧键均相裂解的热力学和动力学摘要:的性质和反应性 自由基阴离子已经通过电化学方法确定了4-氰基苯基烷基醚和萘基烷基醚的含量。在电化学条件下,均解离是唯一观察到的过程。循环伏安法 研究得出的结论是,这一过程是一个循序渐进的过程,最初是 自由基阴离子通过缓慢的一级反应裂解,然后以DISP1机理进行第二次电子转移。裂解速率常数与反应的标准自由能之间的Marcus型关系导致本征势垒在0.7至0.8eV的范围内。对本征势垒值的分析表明:溶剂 组织代表了适度的贡献, 自由基阴离子(结构性贡献)是总障碍的主要因素。使用这项工作建立的相关性,可以估算出以前未知的萘醚键解离能。DOI:10.1039/b110994d
文献信息
-
Tuneable Hydrogenation of Nitriles into Imines or Amines with a Ruthenium Pincer Complex under Mild Conditions作者:Jong-Hoo Choi、Martin H. G. PrechtlDOI:10.1002/cctc.201403047日期:2015.3selective hydrogenation of aromatic and aliphatic nitriles into amines and imines is described. Using a ruthenium pincer complex, the selectivity towards amines or imines can be controlled by simple parameter changes. The reactions are conducted under very mild conditions between 50–100 °C at 0.4 MPa H2 pressure without any additives at low catalytic loadings of 0.5–1 mol %, which results in quantitative conversions
-
[EN] COMPOUNDS, SALTS THEREOF AND METHODS FOR TREATMENT OF DISEASES<br/>[FR] COMPOSÉS, SELS CORRESPONDANTS ET MÉTHODES POUR LE TRAITEMENT DE MALADIES申请人:ACADIA PHARM INC公开号:WO2019040107A1公开(公告)日:2019-02-28The present disclosure relates to compounds according to Formula (I), useful for treating diseases.本公开涉及按照式(I)的化合物,用于治疗疾病。
-
The reductive deaminative conversion of nitriles to alcohols using <i>para</i>-formaldehyde in aqueous solution作者:Ghazal Tavakoli、Martin H. G. PrechtlDOI:10.1039/c9cy01484e日期:——We report herein, for the first time, the application of para-formaldehyde (pFA) to the reductive deamination of both aliphatic and aromatic nitriles in aqueous solution under transfer hydrogenation conditions. A broad range of primary alcohols have been synthesized selectively with very good to excellent yields under the optimized conditions. The study disclosed that the air-stable, inexpensive and
-
AMINO-PYRIDINE DERIVATIVES AS S1P1 /EDG1 RECEPTOR AGONISTS
-
Visible‐Light Promoted C–O Bond Formation with an Integrated Carbon Nitride–Nickel Heterogeneous Photocatalyst作者:Arjun Vijeta、Carla Casadevall、Souvik Roy、Erwin ReisnerDOI:10.1002/anie.202016511日期:2021.4.6photo/Ni dual catalytic cross‐coupling reactions. The dual catalytic Ni‐mpg‐CNx is demonstrated for C–O coupling between aryl halides and aliphatic alcohols under mild condition. The reaction affords the ether product in good‐to‐excellent yields (60–92 %) with broad substrate scope, including heteroaryl and aryl halides bearing electron‐withdrawing, ‐donating and neutral groups. The heterogeneous Ni‐mpg‐CNx
表征谱图
-
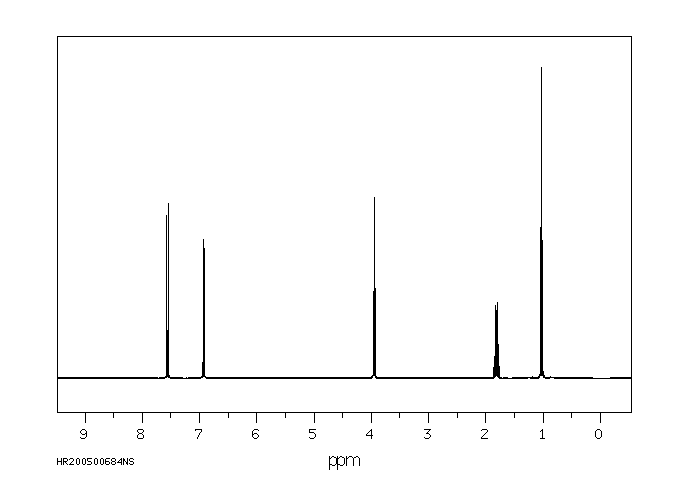
氢谱1HNMR
-
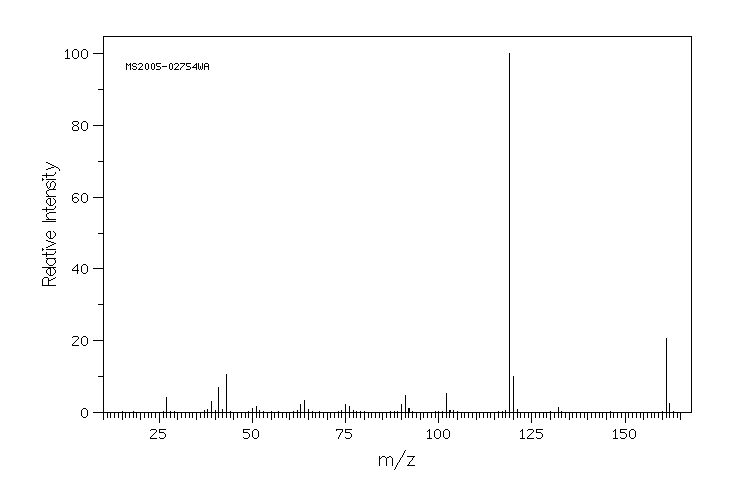
质谱MS
-
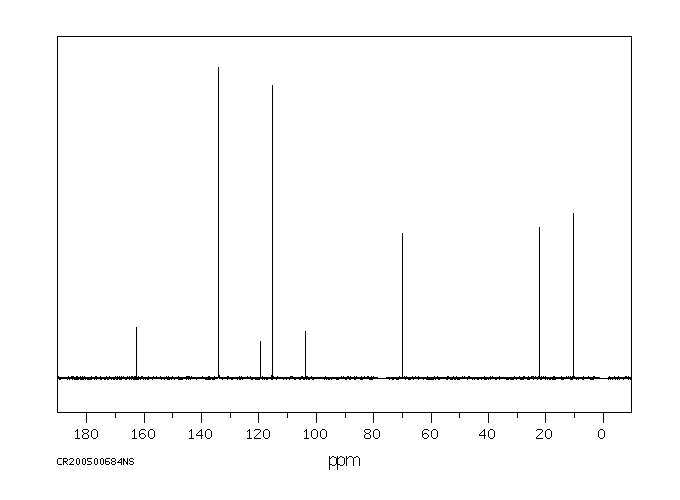
碳谱13CNMR
-
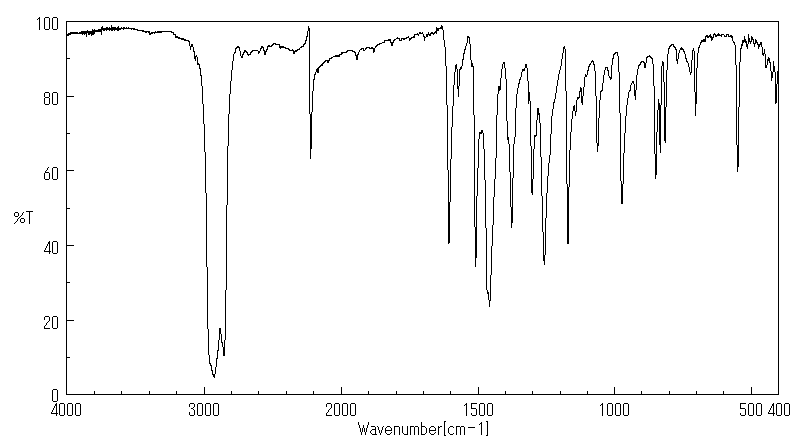
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯