勒胖停 | 155-58-8
中文名称
勒胖停
中文别名
土大黄苷;大黄素荧光指示剂;土大黄素.食用大黄素
英文名称
rhaponticin
英文别名
3,3',5-trihydroxy-4'-methoxystilbene 3-O-β-D-glucoside;rhapontigenin-3-O-glucoside;rhapontin;UNPD24149;4'-methoxy-3,3',5-stilbenetriol 3-glucoside;(2S,3R,4S,5S,6R)-2-[3-hydroxy-5-[(E)-2-(3-hydroxy-4-methoxyphenyl)ethenyl]phenoxy]-6-(hydroxymethyl)oxane-3,4,5-triol
CAS
155-58-8
化学式
C21H24O9
mdl
——
分子量
420.416
InChiKey
GKAJCVFOJGXVIA-DXKBKAGUSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:236-240 °C
-
比旋光度:D32 -59.5° (acetone)
-
沸点:457.61°C (rough estimate)
-
密度:1.2828 (rough estimate)
-
溶解度:可溶于丙酮(少许)、DMSO(少许)、甲醇(少许)
-
LogP:0.680 (est)
计算性质
-
辛醇/水分配系数(LogP):0.5
-
重原子数:30
-
可旋转键数:6
-
环数:3.0
-
sp3杂化的碳原子比例:0.33
-
拓扑面积:149
-
氢给体数:6
-
氢受体数:9
安全信息
-
危险品标志:Xi
-
安全说明:S24/25
-
危险类别码:R36/38
-
WGK Germany:3
-
海关编码:29389090
-
储存条件:请将密封保存。保存为Markdown格式。
SDS
| Name: | Rhapontin, 95% Material Safety Data Sheet |
| Synonym: | 4'Methoxy-3,3',5-stilbenetriol-3-glucoside |
| CAS: | 155-58-8 |
Synonym: 4'Methoxy-3,3',5-stilbenetriol-3-glucoside
SECTION 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 155-58-8 | Rhapontin | 95% | 205-845-0 |
Risk Phrases: None Listed.
SECTION 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW The toxicological properties of this material have not been fully investigated. Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
None
SECTION 4 - FIRST AID MEASURES
Eyes:
Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid if irritation develops or persists. Flush skin with plenty of soap and water.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
Remove from exposure and move to fresh air immediately. Get medical aid if cough or other symptoms appear.
Notes to Physician:
SECTION 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
SECTION 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Avoid generating dusty conditions.
SECTION 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
SECTION 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low. Exposure Limits CAS# 155-58-8: Personal Protective Equipment
Eyes:
Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
SECTION 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: light yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 236-238 deg C dec
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C21H24O9
Molecular Weight: 420.42
SECTION 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Will not occur.
SECTION 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 155-58-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Rhapontin - Not listed by ACGIH, IARC, or NTP.
SECTION 12 - ECOLOGICAL INFORMATION
SECTION 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations.
SECTION 14 - TRANSPORT INFORMATION IATA Not regulated as a hazardous material. IMO Not regulated as a hazardous material. RID/ADR Not regulated as a hazardous material.
SECTION 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases: S 24/25 Avoid contact with skin and eyes. WGK (Water Danger/Protection) CAS# 155-58-8: No information available. Canada None of the chemicals in this product are listed on the DSL/NDSL list. CAS# 155-58-8 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 155-58-8 is not listed on the TSCA inventory. It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
MSDS Creation Date: 9/02/1997 Revision #2 Date: 3/18/2003 The information above is believed to be accurate and represents the best information currently available to us. However, we make no warranty of merchantability or any other warranty, express or implied, with respect to such information, and we assume no liability resulting from its use. Users should make their own investigations to determine the suitability of the information for their particular purposes. In no way shall the company be liable for any claims, losses, or damages of any third party or for lost profits or any special, indirect, incidental, consequential or exemplary damages, howsoever arising, even if the company has been advised of the possibility of such damages.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 脱氧土大黄苷 (E)-3-(β-D-glucopyranosyloxy)-5-hydroxy-4'-methoxystilbene 30197-14-9 C21H24O8 404.417 —— Rhaponticin 6''-O-(E)-p-coumarate 124530-57-0 C30H30O11 566.562 —— rhaponticin 2''-O-p-coumarate 94356-25-9 C30H30O11 566.562 土大黄苷6''-O-没食子酸酯 rhaponticin 6"-gallate 94356-23-7 C28H28O13 572.523 —— rhaponticin 2"-gallate 94356-24-8 C28H28O13 572.523 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— cis-3,3',5-trihydroxy-4'-methoxystilbene 3-O-β-D-glucopyranoside 94356-30-6 C21H24O9 420.416 —— (2S,3R,4S,5R,6R)-2-[3-[(E)-2-(3,4-dimethoxyphenyl)ethenyl]-5-methoxyphenoxy]-3,4,5-trimethoxy-6-(methoxymethyl)oxane 266997-55-1 C27H36O9 504.577 —— rhaponticin 1403836-09-8 C21H25NO8 419.431
反应信息
-
作为反应物:参考文献:名称:Stilbene glycosides from Guibourtia tessmannii摘要:从Guibourtia tessmannii植物的茎 Bark中分离获得了顺式3,4-二甲氧基5-核糖糖基间苯二酚、拉普托因和匹死。DOI:10.1016/0031-9422(94)00915-g
-
作为产物:参考文献:名称:A Short Synthesis of Rhaponticin and its 3”-Fluoroanalog via a Wittig/Heck-Mizoroki Route摘要:胡薄荷次苷及其3”-氟类似物已从易得的起始原料中制备得到。这些合成中的关键步骤是使用碳酸银介导的糖苷化反应,用于选择性地形成β-糖苷键。在三乙醇胺中通过钯醋酸盐催化的Heck-Mizoroki反应,在同时去保护糖类的情况下,建立了芪类化合物的(E)构型。DOI:10.1515/znb-2011-0314
文献信息
-
31P-N.m.r. spectroscopy in wood chemistry. Phosphite derivatives of carbohydrates作者:Yuri Archipov、Dimitris S. Argyropoulos、Henry Bolker、Cyril HeitnerDOI:10.1016/0008-6215(91)80005-8日期:1991.11and model compounds for lignin-carbohydrate complexes were reacted with 1,3,2-dioxaphospholanyl chloride, and the 31P-n.m.r. spectra of the derivatives were obtained in order to correlate the signals with the structural details of the compounds. The derivatives of most of the carbohydrates exhibited spectra containing well resolved signals in the range 138-132 p.p.m., and the number of lines accorded摘要使选定的无环和环状多元醇,单糖,二糖,高级寡糖和木质素-碳水化合物配合物的模型化合物与1,3,2-二氧杂磷酰氯酰氯反应,得到衍生物的31P-nmr光谱以使它们相互关联。表示化合物的结构细节。大多数碳水化合物的衍生物显示出的光谱包含在138-132 ppm范围内的分辨良好的信号,并且谱线数与相应化合物中的羟基数相符。单糖衍生物中C-1处磷原子的化学位移值是所涉及的端基异构体的特征。由于完全分解的光谱分别具有糖和木质素相关部分的特征信号,获得了相关模型化合物的一部分,该方法似乎非常适合于对木质素-碳水化合物复合物衍生的分子进行结构解析。对环糊精(环麦芽六-,庚-和八糖)进行了衍生化处理,检查后得出的光谱彼此之间存在显着差异。
-
Antioxidant constituents from rhubarb: structural requirements of stilbenes for the activity and structures of two new anthraquinone glucosides作者:Hisashi Matsuda、Toshio Morikawa、Iwao Toguchida、Ji-Young Park、Shoichi Harima、Masayuki YoshikawaDOI:10.1016/s0968-0896(00)00215-7日期:2001.1extracts from five kinds of rhubarb were found to show scavenging activity for DPPH radical and .O2-. Two new anthraquinone glucosides were isolated from the rhizome of Rheum undulatum L. together with two anthraquinone glucosides, a naphthalene glucoside, and 10 stilbenes. In the screening test for radical scavenging activity of rhubarb constituents, stilbenes and a naphthalene glucoside showed activity发现从5种大黄中提取的甲醇对DPPH自由基和.O2-具有清除活性。从大黄(Rheum undulatum L.)的根茎中分离出两个新的蒽醌葡糖苷,以及两个蒽醌葡糖苷,萘葡糖苷和10个对苯二酚。在对大黄成分的自由基清除活性的筛选试验中,丁苯二酚和萘葡萄糖苷具有活性,而蒽醌和番石榴苷则没有活性。此外,大多数对苯二酚通过叔丁基氢过氧化物抑制红细胞膜的脂质过氧化。对各种相关化合物的清除作用的详细研究表明以下结构要求;1)酚羟基对显示活性至关重要;2)没食子酰基部分增强活性;3)葡糖苷部分降低活性;4)二氢二苯乙烯衍生物保持对DPPH自由基的清除活性,但它们对.O2-的活性较弱。另外,具有3-羟基和4'-甲氧基的几个对苯二酚抑制黄嘌呤氧化酶。
-
USE OF HYDROXYSTILBENES FOR DYEING, READY-TO-USE COMPOSITION CONTAINING THEM AND DYEING PROCESS申请人:——公开号:US20020016998A1公开(公告)日:2002-02-14The invention relates to the use of hydroxystilbenes for dyeing, to ready-to-use dye compositions comprising them, to a dyeing process using them and to a multi-compartment device containing the compositions used in the processes of the invention.
-
USE OF SARMENTINE AND ITS ANALOGS FOR CONTROLLING PLANT PESTS申请人:Huang Huazhang公开号:US20110021358A1公开(公告)日:2011-01-27Methods and compositions for controlling plant pests, particularly weeds and/or plant phytopathogens using sarmentine and/or analogs thereof are disclosed.揭示了利用沙曼汀和/或其类似物控制植物害虫,特别是杂草和/或植物病原体的方法和组合物。
-
[EN] STREPTOCOCCUS MUTANS GLUCOSYL TRANSFERASE INHIBITORS FOR DENTAL CARIES THERAPY<br/>[FR] INHIBITEURS DE GLUCOSYLE TRANSFÉRASE DE STREPTOCOCCUS MUTANS POUR LA THÉRAPIE DES CARIES DENTAIRES申请人:UAB RESEARCH FOUNDATION公开号:WO2019195430A1公开(公告)日:2019-10-10The present invention is related to the inhibition of the formation of Streptococci biofilms through the inhibition of glucosyl transferase (Gtf). Compounds, compositions and methods for inhibiting Streptococcus biofilm formation, as well as for preventing, inhibiting and/or treating the formation of dental caries, and methods of identifying compounds that prevent, inhibit and/or treat the formation of dental caries are provided.
表征谱图
-
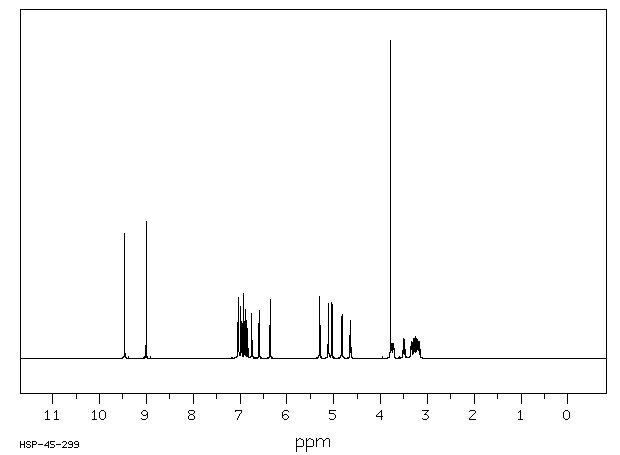
氢谱1HNMR
-
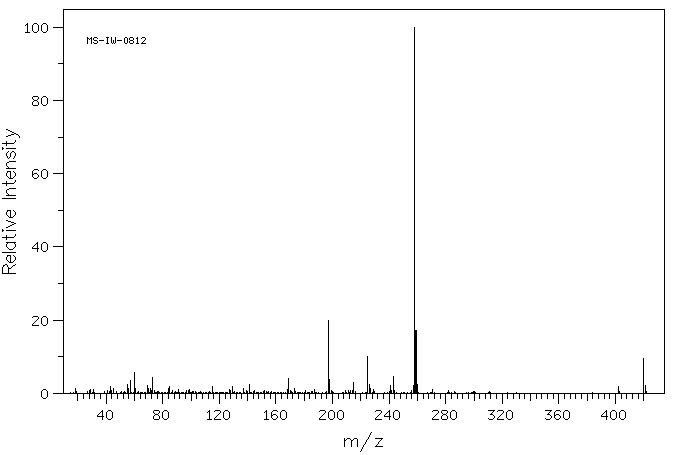
质谱MS
-
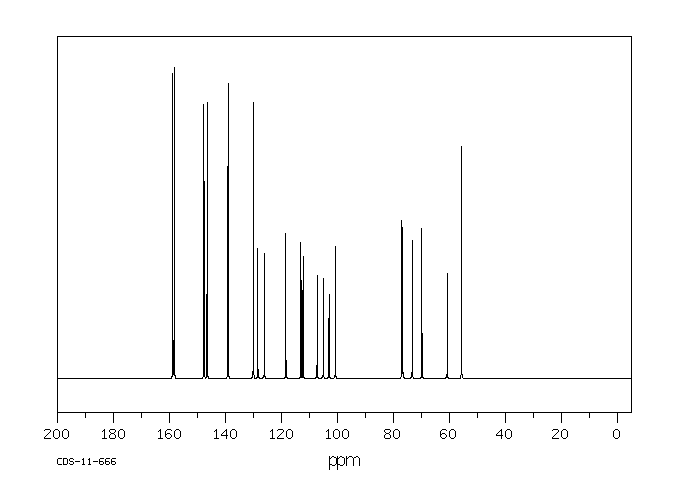
碳谱13CNMR
-
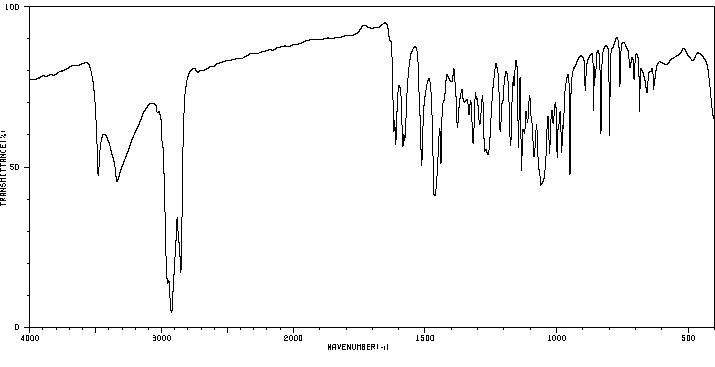
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(E,Z)-他莫昔芬N-β-D-葡糖醛酸
(E/Z)-他莫昔芬-d5
(4S,5R)-4,5-二苯基-1,2,3-恶噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S,5R,5''R)-2,2''-(1-甲基亚乙基)双[4,5-二氢-4,5-二苯基恶唑]
(4R,5S)-4,5-二苯基-1,2,3-恶噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4R,4''R,5S,5''S)-2,2''-(1-甲基亚乙基)双[4,5-二氢-4,5-二苯基恶唑]
(1R,2R)-2-(二苯基膦基)-1,2-二苯基乙胺
鼓槌石斛素
黄子囊素
高黄绿酸
顺式白藜芦醇三甲醚
顺式白藜芦醇
顺式己烯雌酚
顺式-白藜芦醇3-O-beta-D-葡糖苷酸
顺式-桑皮苷A
顺式-曲札芪苷
顺式-二苯乙烯
顺式-beta-羟基他莫昔芬
顺式-a-羟基他莫昔芬
顺式-3,4',5-三甲氧基-3'-羟基二苯乙烯
顺式-1-(3-甲基-2-萘基)-2-(2-萘基)乙烯
顺式-1,2-双(三甲基硅氧基)-1,2-双(4-溴苯基)环丙烷
顺式-1,2-二苯基环丁烷
顺-均二苯乙烯硼酸二乙醇胺酯
顺-4-硝基二苯乙烯
顺-1-异丙基-2,3-二苯基氮丙啶
非洲李(PRUNUSAFRICANA)树皮提取物
阿非昔芬
阿里可拉唑
阿那曲唑二聚体
阿托伐他汀环氧四氢呋喃
阿托伐他汀环氧乙烷杂质
阿托伐他汀环(氟苯基)钠盐杂质
阿托伐他汀环(氟苯基)烯丙基酯
阿托伐他汀杂质D
阿托伐他汀杂质94
阿托伐他汀杂质7
阿托伐他汀杂质5
阿托伐他汀内酰胺钠盐杂质
阿托伐他汀中间体M4
阿奈库碘铵
锌(II)(苯甲醛)(四苯基卟啉)
银松素
铜酸盐(5-),[m-[2-[2-[1-[4-[2-[4-[[4-[[4-[2-[4-[4-[2-[2-(羧基-kO)苯基]二氮烯基-kN1]-4,5-二氢-3-甲基-5-(羰基-kO)-1H-吡唑-1-基]-2-硫代苯基]乙烯基]-3-硫代苯基]氨基]-6-(苯基氨基)-1,3,5-三嗪-2-基]氨基]-2-硫代苯基]乙烯基]-3-硫代
铒(III) 离子载体 I
铀,二(二苯基甲酮)四碘-
钾钠2,2'-[(E)-1,2-乙烯二基]二[5-({4-苯胺基-6-[(2-羟基乙基)氨基]-1,3,5-三嗪-2-基}氨基)苯磺酸酯](1:1:1)
钠{4-[氧代(苯基)乙酰基]苯基}甲烷磺酸酯
钠;[2-甲氧基-5-[2-(3,4,5-三甲氧基苯基)乙基]苯基]硫酸盐
钠4-氨基二苯乙烯-2-磺酸酯