5-氯-3-甲酰基-1H-吲哚-2-羧酸乙酯 | 43142-76-3
中文名称
5-氯-3-甲酰基-1H-吲哚-2-羧酸乙酯
中文别名
5-氯-3-醛基-1H-吲唑-2-甲酸乙酯
英文名称
ethyl 5-chloro-3-formyl-1H-indole-2-carboxylate
英文别名
——
CAS
43142-76-3
化学式
C12H10ClNO3
mdl
MFCD02257705
分子量
251.669
InChiKey
JSJQTSYYPLBOFH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:458.0±45.0 °C(Predicted)
-
密度:1.399±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:17
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.17
-
拓扑面积:59.2
-
氢给体数:1
-
氢受体数:3
安全信息
-
海关编码:2933990090
-
危险性防范说明:P261,P264,P270,P271,P280,P302+P352,P304+P340,P305+P351+P338,P312,P330,P362,P403+P233,P501
-
危险性描述:H302,H312,H332
-
储存条件:室温
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 5-Chloro-3-formyl-1h-indole-2-carboxylic acid ethyl ester
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 5-Chloro-3-formyl-1h-indole-2-carboxylic acid ethyl ester
CAS number: 43142-76-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C12H10ClNO3
Molecular weight: 251.7
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen chloride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 5-Chloro-3-formyl-1h-indole-2-carboxylic acid ethyl ester
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 5-Chloro-3-formyl-1h-indole-2-carboxylic acid ethyl ester
CAS number: 43142-76-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C12H10ClNO3
Molecular weight: 251.7
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen chloride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5-氯吲哚-2-羧酸乙酯 5-chloro-indole-2-carboxylic acid ethyl ester 4792-67-0 C11H10ClNO2 223.659 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 5-氯-3-甲基-1H-吲哚-2-羧酸乙酯 ethyl 5-chloro-3-methyl-1H-indole-2-carboxylate 16382-20-0 C12H12ClNO2 237.686 5-氯-3-甲酰基-1H-吲哚-2-羧酸 5-chloro-3-formylindole-2-carboxylic acid 380448-07-7 C10H6ClNO3 223.616 5-氯-2-(乙氧基羰基)吲哚-3-羧酸 5-chloro-2-(ethoxycarbonyl)indole-3-carboxylic acid 480450-97-3 C12H10ClNO4 267.669 5-氯-3-甲基-1H-吲哚-2-羧酸 5-chloro-3-methyl-1H-indole-2-carboxylic acid 16381-47-8 C10H8ClNO2 209.632 —— 5-chloro-3-methyl-N-(4-(piperidin-1-yl)phenethyl)-1H-indole-2-carboxamide 1377838-14-6 C23H26ClN3O 395.932
反应信息
-
作为反应物:描述:5-氯-3-甲酰基-1H-吲哚-2-羧酸乙酯 在 sodium chlorite 、 氨基磺酸 、 盐酸 作用下, 以 水 、 叔丁醇 为溶剂, 反应 8.0h, 以43%的产率得到5-氯-2-(乙氧基羰基)吲哚-3-羧酸参考文献:名称:设计,合成和合成顺式-1,2-二氨基环己烷衍生物作为有效的Xa因子抑制剂。第一部分:探索5–6个稠合环作为S1的替代部分摘要:为了优化先前公开的因子Xa(fXa)抑制剂,合成了一系列顺式-1,2-二氨基环己烷衍生物。探索5-6个稠合环作为S1的替代部分,与临床候选化合物A相比,发现了两种化合物具有更高的溶解度和降低的食物效果。在这里,我们描述了一些预期化合物的合成和结构-活性关系(SAR),以及理化性质和药代动力学(PK)谱。DOI:10.1016/j.bmc.2009.10.023
-
作为产物:描述:参考文献:名称:Antithrombotic diaminocyclohexane derivatives摘要:公开号:EP1577301B1
文献信息
-
Discovery of 3-Substituted 1<i>H</i>-Indole-2-carboxylic Acid Derivatives as a Novel Class of CysLT<sub>1</sub>Selective Antagonists作者:Huayan Chen、Hui Yang、Zhilong Wang、Xin Xie、Fajun NanDOI:10.1021/acsmedchemlett.5b00482日期:2016.3.10The indole derivative, 3-((E)-3-((3-((E)-2-(7-chloroquinolin-2yl)vinyl)phenyl)amino)-3-oxoprop-1-en-1-yl )-7-methoxy-1H-indole-2-carboxylic acid (17k), was identified as a novel and highly potent and selective CysLT1 antagonist with IC50 values of 0.0059 +/- 0.0011 and 15 +/- 4 muM for CysLT1 and CysLT2, respectively.
-
Diamine derivatives申请人:Ohta Toshiharu公开号:US20050020645A1公开(公告)日:2005-01-27A compound represented by the general formula (1): Q 1 -Q 2 -T 0 -N(R 1 )-Q 3 -N(R 2 )-T 1 -Q 4 (1) wherein R 1 and R 2 are hydrogen atoms or the like; Q 1 is a saturated or unsaturated, 5- or 6-membered cyclic hydrocarbon group which may be substituted, or the like; Q 2 is a single bond or the like; Q 3 is a group in which Q 5 is an alkylene group having 1 to 8 carbon atoms, or the like; and T 0 and T 1 are carbonyl groups or the like; a salt thereof, a solvate thereof, or an N-oxide thereof. The compound is useful as an agent for preventing and/or treating cerebral infarction, cerebral embolism, myocardial infarction, angina pectoris, pulmonary infarction, pulmonary embolism, Buerger's disease, deep venous thrombosis, disseminated intravascular coagulation syndrome, thrombus formation after valve or joint replacement, thrombus formation and reocclusion after angioplasty, systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), thrombus formation during extracorporeal circulation, or blood clotting upon blood drawing.通用式(1)表示的化合物: Q1-Q2-T0-N(R1)-Q3-N(R2)-T1-Q4(1) 其中R1和R2是氢原子或类似物;Q1是饱和或不饱和的、5-或6-成员环烃基,可以被取代,或类似物;Q2是单键或类似物;Q3是一个基团,其中Q5是具有1至8个碳原子的烷基基团,或类似物;T0和T1是羰基团或类似物;其盐、溶剂合物或N-氧化物。 该化合物可用作预防和/或治疗脑梗死、脑栓塞、心肌梗死、心绞痛、肺梗死、肺栓塞、布尔格病、深静脉血栓形成、弥散性血管内凝血综合征、瓣膜或关节置换后的血栓形成、血管成形术后的血栓形成和再闭塞、全身性炎症反应综合征(SIRS)、多器官功能障碍综合征(MODS)、体外循环期间的血栓形成,或抽血时的血液凝结。
-
Design, synthesis, and biological evaluation of novel EGFR inhibitors containing 5-chloro-3-hydroxymethyl-indole-2-carboxamide scaffold with apoptotic antiproliferative activity作者:Fatma A.M. Mohamed、Hesham A.M. Gomaa、O.M. Hendawy、Asmaa T. Ali、Hatem S. Farghaly、Ahmed M. Gouda、Ahmed H. Abdelazeem、Mostafa H. Abdelrahman、Laurent Trembleau、Bahaa G.M. YoussifDOI:10.1016/j.bioorg.2021.104960日期:2021.7New EGFR inhibitor series of fifteen 5-chloro-3-hydroxymethyl-indole-2-carboxamide derivatives has been designed, synthesized, and tested for antiproliferative activity against a panel of cancer cell lines. The results showed that p-substituted phenethyl derivatives 10, 11, 13, 15 and 17-19 showed superior antiproliferative activity compared to their m-substituted counterparts 12, 14, 16 and 20. Compounds15 种 5-氯-3-羟甲基-吲哚-2-甲酰胺衍生物的新型 EGFR 抑制剂系列已被设计、合成并测试其对一组癌细胞系的抗增殖活性。结果表明,p取代的苯乙基衍生物10,11,13,15和17-19相比,其表现出优异的抗增殖活性的米-取代的对应物12,14,16和20。化合物15,16,19和20显示出有希望的 EGFR 抑制活性以及半胱天冬酶 3 水平的增加。化合物15和19增加了 caspase-8 和 9 的水平,以及诱导 Bax 和降低 Bcl-2 蛋白水平。化合物19在前 G1 和 G2/M 阶段表现出细胞周期停滞。与 EGFR 活性位点对接研究的结果表明,与厄洛替尼相比,具有更高结合亲和力的新化合物具有很强的拟合能力。
-
Synthesis and Biological Activity of Functionalized Indole-2-carboxylates, Triazino- and Pyridazino-indoles作者:Adel A. El-Gendy、Mohamed M. Said、Nagat Ghareb、Yasser M. Mostafa、El Sayed H. El-AshryDOI:10.1002/ardp.200700161日期:2008.54]triazino[4,5‐a]indol‐1(2H)‐one 14. Vilsmeier–Haack formylation of 7–9 gave ethyl 3‐formyl‐substituted‐1H‐indole‐2‐carboxylates 15–17 whose 2,2′‐((5‐chloro‐2‐(ethoxycarbonyl)‐1H‐indol‐3‐yl)methylene)bis‐(sulfanediyl) diacetic acid 18 was prepared. The reaction of 15 and 16 with substituted anilines by conventional and microwave methods gave ethyl 3‐(N‐aryliminomethyl)‐5‐halo‐1H‐indole‐2‐carboxylates 19–29.芳基肼与丙酮酸乙酯缩合得到相应的腙4-6;Fischer 吲哚化导致取代的 - 1H - 吲哚 - 2 - 羧酸乙酯 7-9。这些化合物与甲醛和吗啉的曼尼希反应生成 3-(吗啉甲基)-取代的 1H-吲哚-2-羧酸乙酯 10-12。5,7-二氯-1H-indole-2-carbohydrazide 13 在 DMF 中与原甲酸甲酯环化得到 6,8-二氯 [1,2,4] 三嗪基 [4,5-a] indole-1 (2H) -One 14. Vilsmeier – Haack 甲酰化 7-9 得到 3-甲酰基-取代的-1H-吲哚-2-羧酸乙酯 15-17,其 2,2'-((5-氯-2-(乙氧基羰基)-1H-制备了吲哚-3-基)亚甲基)双(硫烷二基)二乙酸18。15和16与取代苯胺通过常规和微波方法反应得到3-(N-芳基亚氨基甲基)-5-卤代-1H-吲哚-2-羧酸乙酯19-29。在 19-25 与
-
Design of C3-Alkenyl-Substituted 2-Indolylmethanols for Catalytic Asymmetric Interrupted Nazarov-Type Cyclization作者:Cong-Shuai Wang、Jia-Le Wu、Can Li、Lin-Zhi Li、Guang-Jian Mei、Feng ShiDOI:10.1002/adsc.201701521日期:2018.3.1The C3‐alkenyl‐substituted 2‐indolylmethanols have been designed as a new class of substrates for catalytic asymmetric interrupted Nazarov‐type cyclizations. In the presence of chiral phosphoric acid as a mild chiral Brønsted acid, the interrupted Nazarov‐type cyclization of C3‐alkenyl‐substituted 2‐indolylmethanols with nucleophiles occurred smoothly to construct cyclopenta[b]indole frameworks in
表征谱图
-
氢谱1HNMR
-
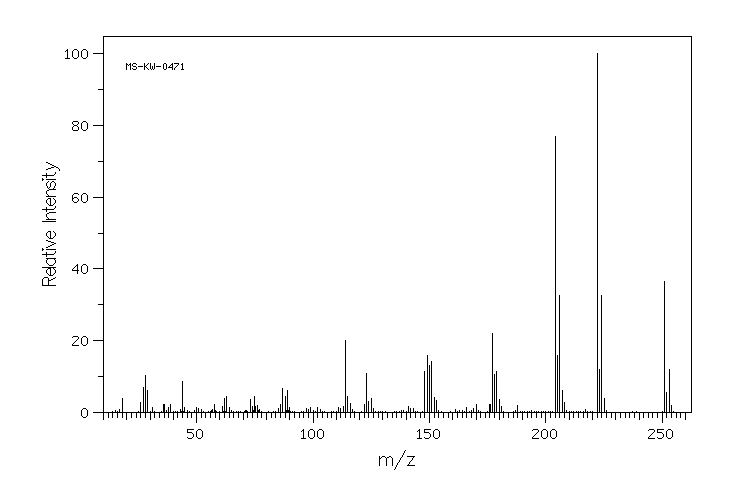
质谱MS
-
碳谱13CNMR
-
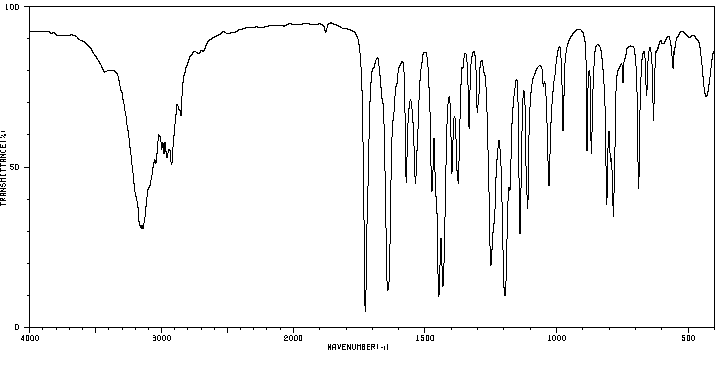
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Z)-3-[[[2,4-二甲基-3-(乙氧羰基)吡咯-5-基]亚甲基]吲哚-2--2-
(S)-(-)-5'-苄氧基苯基卡维地洛
(R)-(+)-5'-苄氧基卡维地洛
(R)-卡洛芬
(N-(Boc)-2-吲哚基)二甲基硅烷醇钠
(E)-2-氰基-3-(5-(2-辛基-7-(4-(对甲苯基)-1,2,3,3a,4,8b-六氢环戊[b]吲哚-7-基)-2H-苯并[d][1,2,3]三唑-4-基)噻吩-2-基)丙烯酸
(4aS,9bR)-6-溴-2,3,4,4a,5,9b-六氢-1H-吡啶并[4,3-B]吲哚
(3Z)-3-(1H-咪唑-5-基亚甲基)-5-甲氧基-1H-吲哚-2-酮
(3Z)-3-[[[4-(二甲基氨基)苯基]亚甲基]-1H-吲哚-2-酮
(3R)-(-)-3-(1-甲基吲哚-3-基)丁酸甲酯
(3-氯-4,5-二氢-1,2-恶唑-5-基)(1,3-二氧代-1,3-二氢-2H-异吲哚-2-基)乙酸
齐多美辛
鸭脚树叶碱
鸭脚木碱,鸡骨常山碱
鲜麦得新糖
高氯酸1,1’-二(十六烷基)-3,3,3’,3’-四甲基吲哚碳菁
马鲁司特
马鞭草(VERBENAOFFICINALIS)提取物
马来酸阿洛司琼
马来酸替加色罗
顺式-ent-他达拉非
顺式-1,3,4,4a,5,9b-六氢-2H-吡啶并[4,3-b]吲哚-2-甲酸乙酯
顺式-(+-)-3,4-二氢-8-氯-4'-甲基-4-(甲基氨基)-螺(苯并(cd)吲哚-5(1H),2'(5'H)-呋喃)-5'-酮
靛青二磺酸二钾盐
靛藍四磺酸
靛红联二甲酚
靛红磺酸钠
靛红磺酸
靛红乙烯硫代缩酮
靛红-7-甲酸甲酯
靛红-5-磺酸钠
靛红-5-磺酸
靛红-5-硫酸钠盐二水
靛红-5-甲酸甲酯
靛红
靛玉红衍生物E804
靛玉红3'-单肟5-磺酸
靛玉红-3'-单肟
靛玉红
靛噻
青色素3联己酸染料,钾盐
雷马曲班
雷莫司琼杂质13
雷莫司琼杂质12
雷莫司琼杂质
雷替尼卜定
雄甾-1,4-二烯-3,17-二酮
阿霉素的代谢产物盐酸盐
阿贝卡尔
阿西美辛杂质3