1-丁氧基乙基苯 | 4157-77-1
中文名称
1-丁氧基乙基苯
中文别名
——
英文名称
(1-butoxyethyl)benzene
英文别名
(1-Phenyl-aethyl)-butyl-aether;α-methylbenzyl n-butyl ether;Benzene, (1-butoxyethyl)-;1-butoxyethylbenzene
CAS
4157-77-1
化学式
C12H18O
mdl
——
分子量
178.274
InChiKey
AQGPVCBVZAHVBN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:13
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2909309090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,1'-(氧基二乙亚基)二-苯 bis(1-phenylethyl)ether 93-96-9 C16H18O 226.318 苏合香醇 1-Phenylethanol 98-85-1 C8H10O 122.167 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 丁酸苏合香酯 1-phenylethyl butyrate 3460-44-4 C12H16O2 192.258
反应信息
-
作为反应物:参考文献:名称:苯甲醇和醚的选择性氧化以及苄基四氢吡喃基和三甲基甲硅烷基醚通过四氧化二氮浸渍的活性炭(N2O4/木炭)氧化裂解为它们的羰基化合物摘要:摘要 在室温下,用活性炭(N2O4/木炭)浸渍四氧化二氮将苯甲醇和醚氧化成相应的羰基化合物。三甲基甲硅烷基 (TMS) 和四氢吡喃基 (THP) 醚有效氧化裂解为其相应的羰基化合物也是通过该试剂进行的。在四氢吡喃基醚的存在下,观察到对苄醇和醚以及三甲基甲硅烷基醚的氧化具有高选择性。DOI:10.1081/scc-200058001
-
作为产物:参考文献:名称:氧化亲核取代:烷基硼衍生物的转化摘要:本文描述了一种有效的酰胺化反应。在乙酸铜和三氟化硼的存在下,烷基三氟硼酸钾盐可从腈转化为酰胺。反应的延伸允许形成胺,醚和CC键。DOI:10.1016/j.tet.2011.09.055
-
作为试剂:描述:一氧化碳 、 1-(1-溴乙基)-4-氯苯 在 氢氧化钾 、 dicobalt octacarbonyl 、 1-丁氧基乙基苯 作用下, 以 正丁醇 为溶剂, 35.0 ℃ 、101.32 kPa 条件下, 以47%的产率得到4-(氯甲基)苯乙酸参考文献:名称:Phase-transfer catalysis in cobalt catalyzed carbonylation of secondary benzyl halides摘要:DOI:10.1016/s0022-328x(00)86850-3
文献信息
-
Direct and efficient synthesis of unsymmetrical ethers from alcohols catalyzed by Fe(HSO4)3 under solvent‐free conditions作者:Bashir Nazari Moghadam、Batool Akhlaghinia、Soodabeh RezazadehDOI:10.1007/s11164-015-2098-y日期:2016.2Highly efficient Fe(HSO4)3 catalyzed etherification of primary, secondary and tertiary benzylic alcohols with primary and secondary aliphatic alcohols is reported. The reaction affords unsymmetrical benzyl ethers in good to excellent yields under solvent-free conditions.报道了伯,仲和叔苄醇与伯和仲脂族醇的高效Fe(HSO 4)3催化醚化。该反应在无溶剂条件下以良好或优异的产率提供不对称的苄基醚。
-
Sulfated tungstate as hydroxyl group activator for preparation of benzyl, including <i>p</i>-methoxybenzyl ethers of alcohols and phenols作者:Kamlesh V. Katkar、Sachin D. Veer、Krishnacharya G. AkamanchiDOI:10.1080/00397911.2016.1230218日期:2016.12.1ABSTRACT Sulfated tungstate was found to be an effective heterogeneous and reusable catalyst for hydroxy group activation–mediated preparation of benzylic ethers including p-methoxybenzylic ethers of a wide range of alcohols and phenols under mild reaction conditions. GRAPHICAL ABSTRACT
-
Efficient addition of alcohols, amines and phenol to unactivated alkenes by AuIII or PdII stabilized by CuCl2作者:Xin Zhang、Avelino CormaDOI:10.1039/b714617e日期:——The nucleophilic addition of alcohols, amines and phenol to unactivated alkenes catalyzed by cationic gold and palladium becomes limited due to the fast reduction into metallic gold under reaction conditions. The presence of CuCl2 retards the reduction of AuIII and PdII, strongly increasing the turnover number of gold and palladium catalysts. It is shown that new AuIII–CuCl2 and PdII–CuCl2 catalysts
-
Effective Au(<scp>iii</scp>)–CuCl<sub>2</sub>-catalyzed addition of alcohols to alkenes作者:Xin Zhang、Avelino CormaDOI:10.1039/b706961h日期:——Alkenes can be activated by Au(III) catalysts, and the effective addition of alcohols to alkenes can be carried out under mild conditions with Au(III), provided that catalytic amounts of CuCl(2) are added, which significantly stabilize the cationic Au(III).烯烃可以通过Au(III)催化剂活化,并且可以在温和的条件下使用Au(III)将醇有效地添加到烯烃中,只要添加催化量的CuCl(2),即可显着稳定阳离子Au (III)。
-
Silica-Supported KHSO4: An Efficient System for Activation of Aromatic Terminal Olefins作者:Amrit Goswami、Ram Das、Kuladip Sarma、Madan PathakDOI:10.1055/s-0030-1259041日期:2010.12Potassium hydrogen sulfate adsorbed on chromatography-grade silica gel activates electron-rich aromatic terminal olefins towards nucleophilic attack at the benzylic position by alcohols. Temperature plays a crucial role and facilitates suppressing nucleophilic reaction in favor of dimerization of the terminal olefin.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
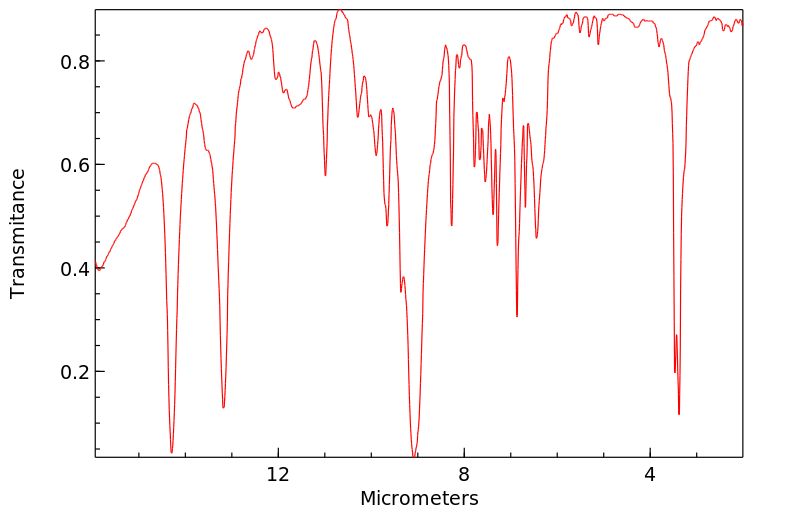
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫