4-硝基苯基乙醛酸乙酯 | 70091-75-7
中文名称
4-硝基苯基乙醛酸乙酯
中文别名
对硝基苯基乙醛酸乙酯
英文名称
ethyl 2-(4-nitrophenyl)-2-oxoacetate
英文别名
ethyl 4-nitrobenzoylformate;ethyl 4-nitrophenylglyoxylate;ethyl 4-nitro-α-oxo-benzeneacetate
CAS
70091-75-7
化学式
C10H9NO5
mdl
——
分子量
223.185
InChiKey
ZFCXKZCKJZFZGR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:40-42°C
-
沸点:152-157°C 0,5mm
-
闪点:152-157°C/0.5mm
-
稳定性/保质期:
远离氧化物。
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:16
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:89.2
-
氢给体数:0
-
氢受体数:5
安全信息
-
安全说明:S22,S24/25
-
海关编码:2918300090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:存放在密封容器内,并置于阴凉、干燥处。储存地点须远离氧化剂。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 对硝基苯乙酸乙酯 ETHYL 4-NITROPHENYLACETATE 5445-26-1 C10H11NO4 209.202 对硝基苯乙酮 (4-nitrophenyl)ethanone 100-19-6 C8H7NO3 165.148 对硝基苯乙酸 4-nitrobenzeneacetic acid 104-03-0 C8H7NO4 181.148 —— ethyl 2-chloro-2-(4-nitrophenyl)ethanoate 102333-76-6 C10H10ClNO4 243.647 2,2-二溴-1-(4-硝基苯基)-1-乙酮 2,2-dibromo-1-(4-nitro-phenyl)-ethanone 21566-36-9 C8H5Br2NO3 322.941 (R)-4-硝基扁桃酸 2-hydroxy-2-(4-nitrophenyl)acetic acid 10098-39-2 C8H7NO5 197.147 对硝基苯甲酰醋酸乙酯 ethyl 4-nitrobenzoylacetate 838-57-3 C11H11NO5 237.212 2-硝基-1-(4-硝基苯基)乙酮 p-Nitrobenzoylnitromethane 46417-99-6 C8H6N2O5 210.146 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-硝基苯乙醛酸 4-nitrophenylglyoxylic acid 14922-36-2 C8H5NO5 195.131 —— N,N-dimethyl-2-(4-nitrophenyl)-2-oxoacetamide 431059-81-3 C10H10N2O4 222.2
反应信息
-
作为反应物:描述:4-硝基苯基乙醛酸乙酯 在 三乙胺 作用下, 以 二氯甲烷 、 甲苯 为溶剂, 80.0 ℃ 、1.72 MPa 条件下, 反应 18.0h, 生成 ethyl 2-diazo-2-(4-nitrophenyl)acetate参考文献:名称:通过安全连续流产生的重氮酯快速获取α-烷氧基和α-氨基酸衍生物摘要:微反应器:A高效连续流动过程已经被开发用于diazoesters从arylsulfonylhydrazones通过在流福德-史蒂文斯反应的手段的合成。此外,α -烷氧基和α -氨基酸衍生物的范围已被以优良产率通过制备铑(II) -介导ö 氢和氮 ħ插入,而不需要分离或处理潜在的危险重氮物种(见方案)。DOI:10.1002/chem.201101590
-
作为产物:描述:ethyl 2-diazo-2-(4-nitrophenyl)acetate 在 四丁基碘化铵 、 溶剂黄146 作用下, 以 乙腈 为溶剂, 反应 12.0h, 以48%的产率得到4-硝基苯基乙醛酸乙酯参考文献:名称:电化学双电子氧还原反应 (ORR) 诱导 α-重氮酯的有氧氧化摘要:电化学氧还原反应 (ORR) 是在合成化学中引入氧官能团的有力工具。然而,与成熟的单电子氧还原工艺相比,双电子氧还原在电化学合成中的应用研究较少。我们在此介绍了我们在通过双电子氧还原方法原位产生的过氧化氢将 α-重氮酯氧化为 α-酮酯方面的最新进展。在无外源氧化剂和无金属催化剂的电化学条件下,以中等至高产率获得了多种有价值的 α-酮酯产物。DOI:10.1039/d1cc06945d
文献信息
-
Aminimides derived from <i>p</i> -substituted benzoylformic acid ester as thermal/photolatent bases and photoradical initiators作者:Manabu Kirino、Ikuyoshi TomitaDOI:10.1002/pola.26838日期:2013.10were synthesized from 1,1‐dimethylhydrazine, propylene oxide, and p‐substituted benzoylformic acid ester, respectively, and their activity as thermal/photolatent bases and photoradical initiators was studied in detail. Their thermal decomposition activity increased by the electron‐donating substituents on the benzene ring, being in order of 1a (p‐NMe2) > 1b (p‐MeO) > BFI (H) > 1c (p‐NO2). Photolysis1,1-二甲基-1-(2-羟丙基)胺苯甲酰基甲酰亚胺的衍生物(BFI和1a-c)分别由1,1-二甲基肼,环氧丙烷和对位取代的苯甲酰基甲酸酯合成,其活性为详细研究了热/光潜碱和光自由基引发剂。它们的热分解活性通过苯环上的供电子取代基增加,顺序为1a(p- NMe 2)> 1b(p- MeO)> BFI(H)> 1c(p- NO 2)。光解活性也受到取代基的影响,顺序如下:1b> BFI> 1a> 1c。通过使用氨基酰亚胺作为潜在的引发剂,进行了环氧化物/硫醇体系的热和光诱导碱催化聚合,以及乙烯基单体甲基丙烯酸2-羟乙酯的光自由基聚合。它们作为热碱和光碱/自由基引发剂的活性可以分别与它们的热分解和光解活性相关。©2013 Wiley Periodicals,Inc. J. Polym。科学,A部分:Polym。化学 2013,51,4292–4300
-
COMPOSITIONS USEFUL FOR TREATING DISORDERS RELATED TO KIT申请人:Hodous Brian L.公开号:US20150111857A1公开(公告)日:2015-04-23Compounds and compositions useful for treating disorders related to KIT and PDFGR are described herein.这里描述了用于治疗与KIT和PDFGR相关疾病的化合物和组合物。
-
Antiatherosclerotic and hypolipidemic 4-(monoalkylamino)phenyl alkane,申请人:American Cyanamid Company公开号:US04348399A1公开(公告)日:1982-09-07This disclosure describes novel 4-(monoalkylamino)phenyl alkane, alkene and alkyne carbinols, aldehydes, carboxylic acids and derivatives useful as hypolipidemic and antiatherosclerotic agents.
-
Alkylidene Meldrum's Acids as Platforms for the Vinylogous Synthesis of Dihydropyranones作者:Stéphane Wittmann、Thomas Martzel、Cong Thanh Pham Truong、Martial Toffano、Sylvain Oudeyer、Régis Guillot、Chloée Bournaud、Vincent Gandon、Jean‐François Brière、Giang Vo‐ThanhDOI:10.1002/anie.202014489日期:2021.5.10Upon Brønsted base organocatalysis, ketone‐derived alkylidene Meldrum's acids proved to be competent vinylogous platforms able to undergo a formal (4+2) cycloaddition reaction with dihydro‐2,3‐furandione, providing an unprecedented route to 3,6‐dihydropyran‐2‐ones as spiro[4.5]decane derivatives with up to 98 % ee thanks to the commercially available Takemoto catalyst. Preliminary investigation showed
-
Pyrimidine derivatives申请人:Naganuma Kenji公开号:US20060293343A1公开(公告)日:2006-12-28The object of the invention is to provide a novel compound having an inhibitory action on PDE4 activity with fewer side effects. The invention provides a compound represented by the following general formula (1), possible stereoisomers thereof or racemates thereof, pharmacologically acceptable salts thereof, hydrates thereof, solvated compounds thereof, or prodrugs thereof,本发明的目的是提供一种对PDE4活性具有抑制作用且副作用较少的新型化合物。 该发明提供了一种由以下一般式(1)表示的化合物,其可能的立体异构体或外消旋体,其药理学上可接受的盐,水合物,溶剂化合物,或前药。
表征谱图
-
氢谱1HNMR
-
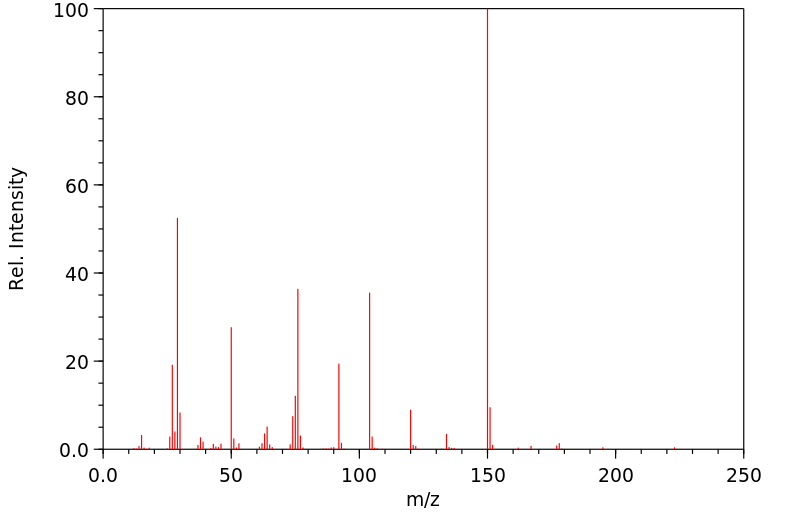
质谱MS
-
碳谱13CNMR
-
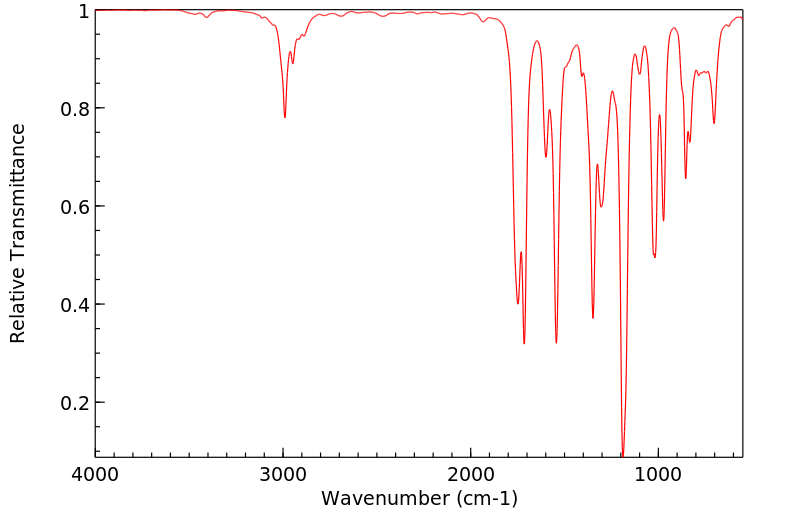
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫