癸酸氯甲酯 | 67317-62-8
中文名称
癸酸氯甲酯
中文别名
——
英文名称
chloromethyl decanoate
英文别名
——
CAS
67317-62-8
化学式
C11H21ClO2
mdl
——
分子量
220.74
InChiKey
UXAJBSZQOZOHEI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:260.1±13.0 °C(Predicted)
-
密度:0.981±0.06 g/cm3(Predicted)
-
保留指数:1470;1479;1481;1483
计算性质
-
辛醇/水分配系数(LogP):4.9
-
重原子数:14
-
可旋转键数:10
-
环数:0.0
-
sp3杂化的碳原子比例:0.91
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
储存条件:室温
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 癸酸甲酯 Methyl decanoate 110-42-9 C11H22O2 186.294 正癸酸 1-decanoic acid 334-48-5 C10H20O2 172.268 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— chloromethyl 10-chlorodecanoate 80418-87-7 C11H20Cl2O2 255.185 —— chloromethyl 9-chlorodecanoate 80418-86-6 C11H20Cl2O2 255.185 —— chloromethyl 7-chlorodecanoate 80418-84-4 C11H20Cl2O2 255.185 —— chloromethyl 8-chlorodecanoate 80418-85-5 C11H20Cl2O2 255.185 —— chloromethyl 6-chlorodecanoate 80418-83-3 C11H20Cl2O2 255.185 —— chloromethyl 5-chlorodecanoate 80418-82-2 C11H20Cl2O2 255.185 —— Chloromethyl 4-chlorodecanoate 80418-81-1 C11H20Cl2O2 255.18 —— iodomethyl decanoate 63379-67-9 C11H21IO2 312.191 —— 3-Chlorodecanoic acid, chloromethyl ester 80418-80-0 C11H20Cl2O2 255.18 —— Chloromethyl 2-chlorodecanoate 80418-79-7 C11H20Cl2O2 255.18 —— Methylendidecanoat 76068-80-9 C21H40O4 356.546 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:1-Alkylcarbonyloxymethyl Prodrugs of 5-Fluorouracil (5-FU): Synthesis, Physicochemical Properties, and Topical Delivery of 5-FU摘要:1-Alkylcarbonyloxymethyl (1-ACOM) prodrugs of 5-fluorouracil (5-FU) have been synthesized and characterized by their solubilities in isopropyl myristate (SIPM) and pH 4.0 buffer (SH2O), by their partition coefficients between isopropyl myristate (IPM) and pH 4.0 buffer (K) and by their abilities to deliver total 5-FU species into (Cs) and through (Ji) hairless mouse skin from an IPM vehicle. All of the prodrugs were much more lipophilic (SIPM) than 5-FU (> 60 times), and two members of the series (alkyl = C1 and C2, acetyl- and propionyloxymethyl) were also more soluble in water than 5-FU. The two more water-soluble members gave larger Ji values than the other members of the series, with C2 exhibiting the best biphasic solubility and the largest Ji value (16 times that of 5-FU). The ability of the 1-ACOM-5-FU prodrugs to deliver total 5-FU species into skin (Cs) was greater than the delivery of 5-FU by 5-FU, except for the last two members of series (alkyl = C7 and C9, octanoyl- and decanoyl-oxymethyl). However, the ratios of normalized Cs to Ji for the series was less than that exhibited by 5-FU, except for C7 and C9. Also, except for C9, significant amounts of intact prodrug as percentages of total 5-FU species were found in the receptor phases during the course of the diffusion cell experiments, ranging from 55% for C1 to 12% for C7.DOI:10.1021/js9702574
-
作为产物:参考文献:名称:[EN] PRODRUGS OF CGRP ANTAGONISTS
[FR] PROMÉDICAMENTS D'ANTAGONISTES DU CGRP摘要:披露了CGRP拮抗剂的前药,治疗CGRP相关疾病的方法,例如偏头痛,通过向需要的患者给予前药,包含前药的药物组合物以及包括药物组合物和使用说明的工具包。公开号:WO2020077038A1
文献信息
-
[EN] ORGANIC COMPOUNDS<br/>[FR] COMPOSÉS ORGANIQUES申请人:INTRA CELLULAR THERAPIES INC公开号:WO2014145192A1公开(公告)日:2014-09-18The invention relates to particular substituted heterocycle fused gamma-carbolines, their prodrugs, in free, pharmaceutically acceptable salt and/or substantially pure form as described herein, pharmaceutical compositions thereof, and methods of use in the treatment of diseases involving 5-HT2A receptor, serotonin transporter (SERT) and/or pathways involving dopamine D2 receptor signaling systems.
-
Erufosine (ErPC3) Cationic Prodrugs as Dual Gene Delivery Reagents for Combined Antitumor Therapy作者:Boris Gaillard、Cendrine Seguin、Jean‐Serge Remy、Françoise Pons、Luc LebeauDOI:10.1002/chem.201903976日期:2019.12.5Sixteen cationic prodrugs of the antitumor alkylphospholipid (APL) erufosine were rationally synthesized to provide original gene delivery reagents with improved cytotoxicity profile. The DNA complexation properties of these cationic lipids were determined and associated transfection rates were measured. Furthermore, the self-assembly properties of the pro-erufosine compounds were investigated and合理地合成了十六种抗肿瘤烷基磷脂(APL)肌苷的阳离子前药,以提供具有改善的细胞毒性特征的原始基因递送试剂。确定了这些阳离子脂质的DNA络合特性,并测量了相关的转染率。此外,研究了芥子油苷化合物的自组装性能,并确定了其临界聚集浓度。在模拟细胞外环境和晚期内体环境的pH条件下,测量了它们的水解稳定性。研究了这些化合物的溶血活性和细胞毒性。在各种细胞系中获得的结果表明,芥子碱的前药显示出与母体抗肿瘤药相似的抗肿瘤活性,但与溶血毒性无关,这是APL的剂量限制性副作用,是其在APL中使用的主要障碍抗癌治疗方案。此外,通过使用由芥酸前药和编码促凋亡蛋白(TRAIL)的质粒DNA制备的脂质复合物,提供了对肿瘤细胞的选择性细胞毒性而非肿瘤细胞具有抗性的证据。这项研究表明,包括良好耐受的芥酸阳离子前药和癌症基因治疗的组合方法在肿瘤治疗中具有重大前景。此外,通过使用由芥酸前药和编码促凋亡蛋白(TRAIL)的
-
POMALIDOMIDE DERIVATIVE AND PREPARATION METHOD THEREFOR申请人:NANJING NORATECH PARMACEUTICALS CO., LTD公开号:US20200199097A1公开(公告)日:2020-06-25Disclosed in the present invention are a Pomalidomide derivative and a preparation method therefor. Specifically, the present invention relates to the Pomalidomide derivative and a stereoisomer thereof, or a pharmaceutically acceptable salt, and applications thereof in the preparation of drugs for treating cancers.
-
Unsaturated Cyclic Ureas as New Nontoxic Biodegradable Transdermal Penetration Enhancers I: Synthesis作者:Ooi Wong、J. Huntington、R. Konishi、J.H. Rytting、T. HiguchiDOI:10.1002/jps.2600771115日期:1988.11A new concept was implemented to reduce the toxicity of some new biodegradable transdermal penetration enhancers. These enhancers consist of 1-alkyl-4-imidazolin-2-one and a long-chain alkyl ester group at the N-3 position. The synthesis involves N-alkylation of the parent compound with soft alkylating agents which were prepared in high yields by an improved method. A phase transfer catalysis technique
-
[EN] COMPOUNDS WITH HIV MATURATION INHIBITORY ACTIVITY<br/>[FR] COMPOSÉS AYANT UNE ACTIVITÉ INHIBITRICE DE LA MATURATION DU VIH申请人:VIIV HEALTHCARE UK NO 5 LTD公开号:WO2019207460A1公开(公告)日:2019-10-31The present invention relates to compound of Formula I or a pharmaceutically acceptable salt thereof (Formula I) wherein R1 is Formula (AA) or Formula (BB) where the squiggly line indicates the point of attachment to the rest of the molecule; R2 is F or Formula (CC) where the squiggly line indicates the point of attachment to the rest of the molecule; R3 is H or CH3; Z is O or is absent; and R4 is -OC1-3alkyl, C1-30alkyl, or -N(CH3)2.
表征谱图
-
氢谱1HNMR
-
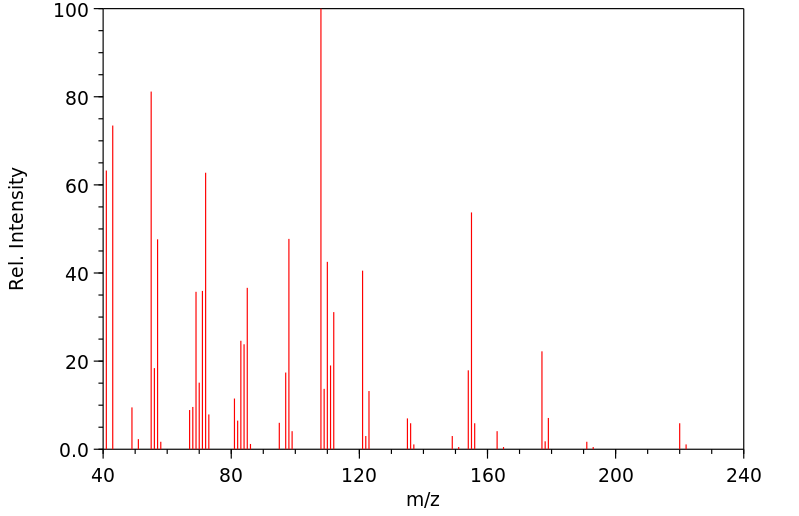
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯