1,2,3,4,5,8-六氢萘 | 36231-13-7
中文名称
1,2,3,4,5,8-六氢萘
中文别名
——
英文名称
1,2,3,4,5,8-hexahydro-naphthalene
英文别名
bicyclo<4.4.0>deca-1(6),3-diene;1,2,3,4,5,8-hexahydronaphthalene;1,2,3,4,5,8-hexahydronaphtalene;1,4-Dihydrotetralin;5,8-dihydrotetralin;dihydrotetralin
CAS
36231-13-7
化学式
C10H14
mdl
——
分子量
134.221
InChiKey
CCLPTDRLVDMCRP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-27 °C
-
沸点:205.5 °C(Press: 737 Torr)
-
密度:0.9446 g/cm3
-
LogP:3.962 (est)
-
保留指数:1147
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:10
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902199090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Aromatization of 1,4-dihydrobenzocycloalkenes, 1,4-dihydronaphthocycloalkenes, and related systems摘要:DOI:10.1021/jo01297a019
-
作为产物:描述:1,2,3,4-四氢萘 在 2-甲基-2-丁醇 、 四甲基氢氧化铵 、 2-(2-ethylhexyl)-6,8,11-tris(4-methoxyphenyl)-1H-peryleno[1,12-efg]isoindole-1,3(2H)-dione 作用下, 以 甲醇 为溶剂, 反应 144.0h, 以38%的产率得到1,2,3,4,5,8-六氢萘参考文献:名称:可见光驱动的有机催化桦木还原反应摘要:Birch 还原是一种强大的合成方法,它使用溶剂化电子将惰性芳烃转化为 1,4-环己二烯有价值的中间体,以构建分子复杂性。桦木还原传统上使用溶解在氨中的碱金属来产生溶剂化电子,用于还原未活化的芳烃,例如苯(Ered < -3.42 V vs. SCE)。光氧化还原催化剂在高度还原应用中越来越受欢迎,但没有报道显示出足以减少苯的还原潜力。在这里,我们引入苯并苝酰亚胺作为新型有机光氧化还原催化剂,用于在环境温度下进行桦木还原,并由市售 LED 的可见光驱动。使用低催化剂负载量(<1 摩尔百分比),在完全无金属的反应中,苯和其他官能化芳烃以中等至良好的产率选择性地转化为 1,4-环己二烯。机理研究表明,这种前所未有的可见光诱导反应是通过有机光氧化还原催化剂利用两个可见光光子的能量影响单个高能化学转化的能力实现的。DOI:10.1021/jacs.0c05899
文献信息
-
Regio- and Stereospecific Synthesis of β-Sulfonamidodisulfides and β-Sulfonamidosulfides from Aziridines using Tetrathiomolybdate as a Sulfur Transfer Reagent作者:Devarajulu Sureshkumar、Thanikachalam Gunasundari、Venkataraman Ganesh、Srinivasan ChandrasekaranDOI:10.1021/jo0624389日期:2007.3.1comprehensive study of a general and effective one-step procedure for the synthesis of β-sulfonamidodisulfides directly from N-tosyl aziridines in a regio- and stereospecific manner under neutral conditions without the use of any Lewis acid or base has been reported. This methodology is extended to the synthesis of an optically pure cyclic seven-membered disulfide 29. Synthesis of a variety of β-sulfonamidosulfides
-
Regio- and Stereoselective Synthesis of Aziridino Epoxides from Cyclic Dienes作者:Devarajulu Sureshkumar、Susama Maity、Srinivasan ChandrasekaranDOI:10.1021/jo052357x日期:2006.2.1for the regio- and stereoslective synthesis of aziridino epoxides from cyclic dienes have been explored. The first strategy involves regiospecific aziridination of cyclic diene derivatives and subsequent epoxidation with m-CPBA to yield cis-aziridino epoxides as major products. The second strategy utilizes regiospecific epoxidation of cyclic diene derivatives followed by Sharpless aziridination to provide
-
Zur Kenntnis des speziellen Charakters der binuclearen Doppelbindung in 4,7-Dihydro-indan作者:E. Giovannini、H. WegmüllerDOI:10.1002/hlca.19590420403日期:——Die Epoxydierungsgeschwindigkeiten von 4,7-Dihydro-indan, 1,2,3,4,5,8-Hexa-hydro-naphtalin und 1,2-Dimethylcyclohexadien-(l, 4) wurden ermittelt.
-
A safe and convenient new procedure for reducing aromatic compounds to birch-type products作者:Robert A. Benkeser、James A. Laugal、Angela RappaDOI:10.1016/s0040-4039(01)81168-0日期:1984.1Aromatic compounds can be reduced by a calcium-amine-t-butyl alcohol system to products which are identical to those obtained by a Birch reduction of the same substrates.
-
Novel Conformationally Locked Inositols: From Aromatics to Annulated Cyclitols作者:Goverdhan Mehta、Ramesh S. Senaiar、Mrinal K. BeraDOI:10.1002/chem.200204650日期:2003.5.23A new family of ring-annulated inositols with "locked" conformations has been designed to deliver a range of these biologically important entities in "unnatural conformations" while retaining their "natural configurations". The simple "tool" of trans ring fusion has been used to "lock" the conformation of the annulated inositols. Short, simple syntheses of a range of these novel cyclitols have been
表征谱图
-
氢谱1HNMR
-
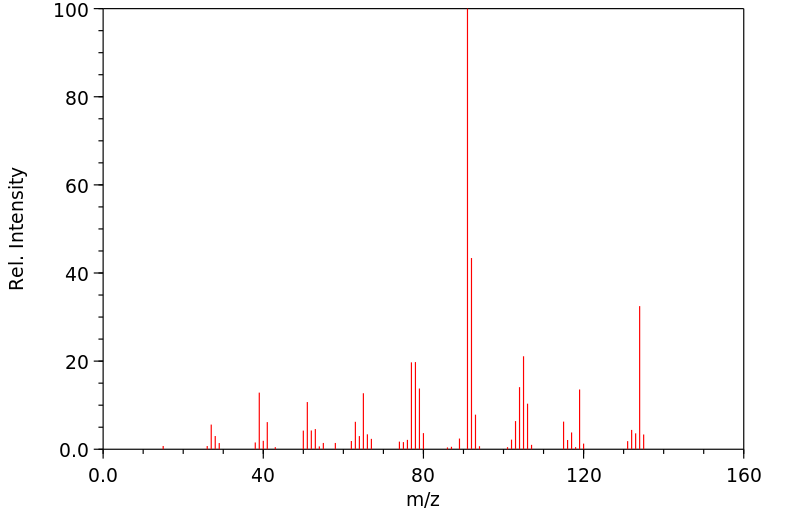
质谱MS
-
碳谱13CNMR
-
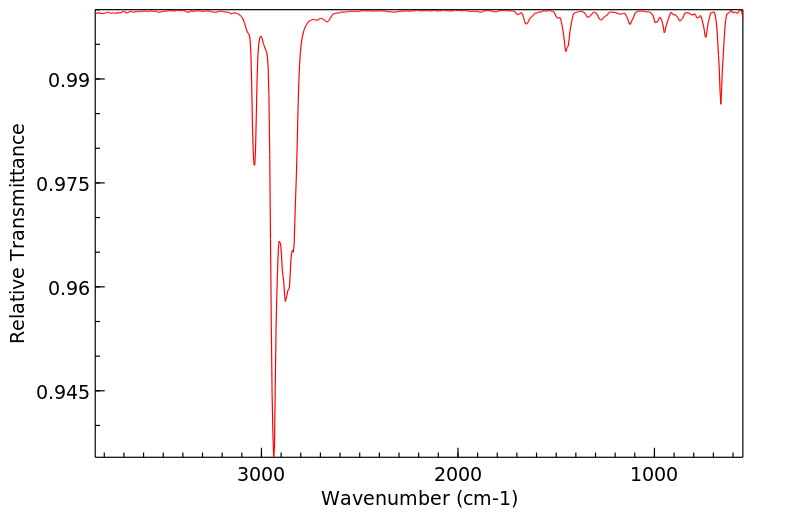
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
降冰片烯
金刚烷-D16
金刚烷
螺戊烷
螺二環己烷
螺[5.6]十二烷
螺[5.5]十一碳-4-烯
螺[5.2]辛-2-烯
螺[4.5]癸烷
螺[4.4]壬-8-烯
螺[3.4]辛烷
螺[3.4]辛-7-烯
螺[3.3]庚-2,5-二烯
螺[2.5]辛烷
螺[2.5]辛-7-烯
螺[2.5]辛-5,7-二烯
螺[2.4]庚-4,6-二烯
螺[2.4]庚-1-烯
螺[2.3]己-1-烯
螺[2.2]戊-1-烯
螺<二环<2.2.2>辛-5-烯-2,1'-环丙烷>
螺<4.4>壬-1,3,7-三烯
螺<4.4>壬-1,3,6,8-四烯
螺(4.4)壬烷
螺(4.4)壬-1,3-二烯
螺(3.4)辛-5,7-二烯
trans-perhydroazulene
萘烷
萘,1,2,3,4,4a,8a-六氢-,顺-
美罗培南中间体F9
篮烷
立方烷
氨基甲硫酸,二甲基-,O,O-(3,3-二甲基1,1-联苯基-2,2-二基)酯
棱晶烷
杜瓦苯
新戊基-1金刚烷
抗氧化剂TH-CPL
庚搭烯
四螺[2.0.2:0.2:0.2:0]十二烷
四螺[2.0.0.0.2.1.1.1]十一烷,顺-
四环己基铅
四环[5.3.0.0<2,6>.0<3,10>]癸-4,8-二烯
四环[5.3.0.0(2,6).0(3,10)]癸烷
四环[4.4.0.02,10.03,7]癸-4,8-二烯
四环[4.2.2.26,5.01,6]十二烷
四环[4.2.0.02,5.03,8]辛烷
四环[3.3.0.02,4.03,6]辛-7-烯
四环<5.3.1.02,6.04,9>十一烷
四环(8.2.2.22,5.26,9)十八碳-1,5,9-三烯
四环(4.1.0.0(2,4).0(3,5))庚烷