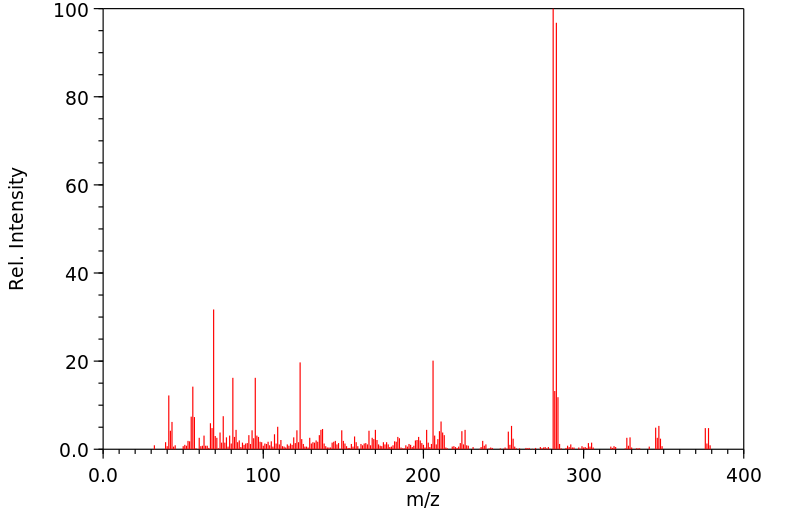
毒理性
苯二氮卓类药物非特异性地与苯二氮卓受体BNZ1结合,介导睡眠,以及与BNZ2结合,影响肌肉松弛、抗惊厥活性、运动协调和记忆。由于认为苯二氮卓受体与γ-氨基丁酸-A (GABAA) 受体相耦合,这通过增加GABA对GABA受体的亲和力来增强GABA的效果。抑制性神经递质GABA结合到该位点时,会打开氯离子通道,导致细胞膜超极化,阻止细胞进一步兴奋。
Benzodiazepines bind nonspecifically to benzodiazepine receptors BNZ1, which mediates sleep, and BNZ2, which affects affects muscle relaxation, anticonvulsant activity, motor coordination, and memory. As benzodiazepine receptors are thought to be coupled to gamma-aminobutyric acid-A (GABAA) receptors, this enhances the effects of GABA by increasing GABA affinity for the GABA receptor. Binding of the inhibitory neurotransmitter GABA to the site opens the chloride channel, resulting in a hyperpolarized cell membrane that prevents further excitation of the cell.
来源:Toxin and Toxin Target Database (T3DB)