3,3'-联吡啶 | 581-46-4
中文名称
3,3'-联吡啶
中文别名
——
英文名称
3,3'-bipyridine
英文别名
3,3′-bipyridine;3,3'-bipyridinyl;3,3'-bipyridyl;3,3’-bipyridyl;3,3′-bpy;3-pyridin-3-ylpyridine
CAS
581-46-4
化学式
C10H8N2
mdl
MFCD00006371
分子量
156.187
InChiKey
OFDVABAUFQJWEZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:68°C
-
沸点:175°C/15mmHg(lit.)
-
密度:1.1614
-
保留指数:1573
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:12
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:25.8
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2933399090
-
储存条件:存放于惰性气体中;避免光照和空气接触。
SDS
3,3'-Bipyridyl Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 3,3'-Bipyridyl
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 3,3'-Bipyridyl
Percent: >98.0%(GC)(T)
CAS Number: 581-46-4
Synonyms: 3,3'-Bipyridine , 3,3'-Dipyridyl
Chemical Formula: C10H8N2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Unsuitable extinguishing Solid streams of water
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
3,3'-Bipyridyl
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a ventilation, local exhaust if vapour or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Light-sensitive, Air-sensitive
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Protective gloves.
Hand protection:
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Colorless - Pale yellow
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
175°C/2kPa
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
Relative density: 1.16
Solubility(ies):
3,3'-Bipyridyl
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
RTECS Number: DW1758000
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
3,3'-Bipyridyl
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 3,3'-Bipyridyl
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 3,3'-Bipyridyl
Percent: >98.0%(GC)(T)
CAS Number: 581-46-4
Synonyms: 3,3'-Bipyridine , 3,3'-Dipyridyl
Chemical Formula: C10H8N2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Unsuitable extinguishing Solid streams of water
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
3,3'-Bipyridyl
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a ventilation, local exhaust if vapour or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Light-sensitive, Air-sensitive
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Protective gloves.
Hand protection:
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Colorless - Pale yellow
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
175°C/2kPa
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
Relative density: 1.16
Solubility(ies):
3,3'-Bipyridyl
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
RTECS Number: DW1758000
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
3,3'-Bipyridyl
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 3,4'-bipyridyl 4394-11-0 C10H8N2 156.187 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-(3'-pyridinyl)pyridine N-oxide 33349-46-1 C10H8N2O 172.186 —— 6-chloro-3,3′-bipyridine 101001-97-2 C10H7ClN2 190.632 —— 2-bromo-3,3'-bipyridine 101002-03-3 C10H7BrN2 235.083 —— 2-chloro-3,3'-bipyridine 101001-94-9 C10H7ClN2 190.632
反应信息
-
作为反应物:描述:参考文献:名称:NEONICOTINE AND ISOMERIC PYRIDYLPIPERIDINES摘要:DOI:10.1021/ja01352a038
-
作为产物:参考文献:名称:Leone; Oliveri, Gazzetta Chimica Italiana, 1885, vol. 15, p. 276摘要:DOI:
文献信息
-
Highly Efficient Monophosphine-Based Catalyst for the Palladium-Catalyzed Suzuki−Miyaura Reaction of Heteroaryl Halides and Heteroaryl Boronic Acids and Esters作者:Kelvin Billingsley、Stephen L. BuchwaldDOI:10.1021/ja068577p日期:2007.3.1active and efficient catalyst system derived from a palladium precatalyst and monophosphine ligands 1 or 2 for the Suzuki-Miyaura cross-coupling reaction of heteroaryl boronic acids and esters has been developed. This method allows for the preparation of a wide variety of heterobiaryls in good to excellent yields and displays a high level of activity for the coupling of heteroaryl chlorides as well as
-
Room-Temperature Suzuki-Miyaura Coupling of Heteroaryl Chlorides and Tosylates作者:Junfeng Yang、Sijia Liu、Jian-Feng Zheng、Jianrong Steve ZhouDOI:10.1002/ejoc.201200918日期:2012.11Suzuki–Miyaura coupling of heteroaryls is an important method for the preparation of compound libraries for medicinal chemistry and materials research. Although many catalysts have been developed, none of them have been generally applicable to the coupling reactions of heteroaryl chlorides and tosylates at room temperature. We discovered that a catalyst combination of Pd(OAc)2 and XPhos (2-dicyclohexylphosphanyl-2′杂芳基的 Suzuki-Miyaura 偶联是制备用于药物化学和材料研究的化合物库的重要方法。尽管已经开发了许多催化剂,但它们都没有普遍适用于杂芳基氯与甲苯磺酸酯在室温下的偶联反应。我们发现 Pd(OAc)2 和 XPhos(2-二环己基膦酰基-2',4',6'-三异丙基联苯)的催化剂组合可以有效地催化这些偶联。除了催化剂的选择,在含水醇溶剂中使用氢氧化物碱对于快速偶联也是必不可少的。这些条件促进了活性催化剂(XPhos)Pd0 的快速释放,并加速了催化循环中的金属转移。大多数杂芳基氯(31 个实例)和甲苯磺酸盐(17 个实例)的主要家族在室温下在几分钟到几小时内达到完全转化。该方法可以很容易地扩大规模以进行克级合成。此外,我们检查了整个反应中偶联伙伴的相对反应性。富电子杂芳基氯化物和甲苯磺酸盐的反应速度比缺电子杂芳基化合物慢,顺序为吲哚、吡咯呋喃、噻吩>吡啶。类似地,缺电子芳基硼酸的反应
-
THIOPHENE-CONTAINING TRIARYLAMINE COMPOUNDS申请人:Xia Chuanjun公开号:US20190165278A1公开(公告)日:2019-05-30Thiophene-containing triarylamine compounds are disclosed. The said compounds have a structure of triarylamine, and connect specific thiophene-containing group. The compounds may be used as hole transporting materials, hole injection materials, or the like in an electroluminescent device. Compared to existing hole transporting materials, hole injection materials, the novel compounds can also offer excellent device performance. An electroluminescent device and a formulation comprising the compounds are also disclosed.
-
COMPOUNDS AND METHODS FOR TREATING OXALATE-RELATED DISEASES申请人:OxaluRx, Inc.公开号:US20210052586A1公开(公告)日:2021-02-25Disclosed herein are compounds and compositions for modulating glycolate oxidase, useful for treating oxalate-related diseases, such as hyperoxaluria, where modulating glycolate oxidase is expected to be therapeutic to a patent in need thereof. Methods of modulating glycolate oxidase activity in a human or animal subject are also provided.
-
Dehydrogenative Synthesis of 2,2′‐Bipyridyls through Regioselective Pyridine Dimerization作者:Shuya Yamada、Takeshi Kaneda、Philip Steib、Kei Murakami、Kenichiro ItamiDOI:10.1002/anie.201814701日期:2019.6.17reactions. The most streamlined approach for the synthesis of 2,2′‐bipyridyls is the dehydrogenative dimerization of unfunctionalized pyridine. Herein, we report on the palladium‐catalyzed dehydrogenative synthesis of 2,2′‐bipyridyl derivatives. The Pd catalysis effectively works with an AgI salt as the oxidant in the presence of pivalic acid. A variety of pyridines regioselectively react at the C2‐positions
表征谱图
-
氢谱1HNMR
-
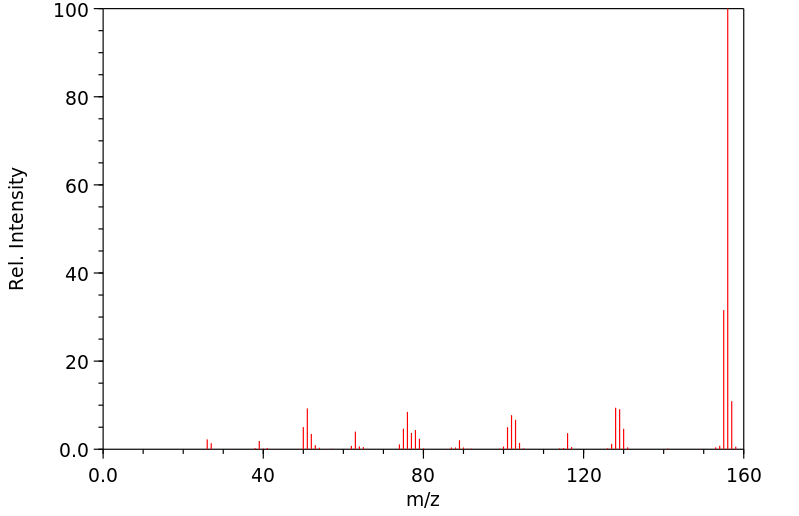
质谱MS
-
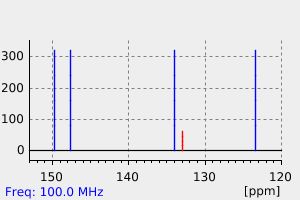
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-