cis-3-氯丙烯酸 | 1609-93-4
中文名称
cis-3-氯丙烯酸
中文别名
顺-3-氯代丙烯酸;顺-3-氯丙烯酸;顺-3-氯代丙烯酸, 98+%
英文名称
3-chloroacrylic acid
英文别名
cis-3-Chloroacrylic acid;(Z)-3-chloroprop-2-enoic acid
CAS
1609-93-4
化学式
C3H3ClO2
mdl
——
分子量
106.509
InChiKey
MHMUCYJKZUZMNJ-UPHRSURJSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:61-62 °C(lit.)
-
沸点:118.27°C (rough estimate)
-
密度:1.2288 (rough estimate)
-
溶解度:可溶于氯仿(轻微)、甲醇(非常轻微)
-
稳定性/保质期:
常温常压下稳定,避免与氧化物、碱和热直接接触。
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:6
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险等级:8
-
危险品标志:C
-
安全说明:S24/25,S26
-
危险类别码:R34
-
WGK Germany:3
-
海关编码:2916190090
-
危险品运输编号:UN 3261 8/PG 2
-
包装等级:III
-
危险类别:8
-
危险性防范说明:P301+P330+P331,P280,P305+P351+P338,P310
-
危险性描述:H314
-
储存条件:密封冷藏
SDS
| Name: | Cis-3-Chloroacrylic Acid 98% Material Safety Data Sheet |
| Synonym: | None known |
| CAS: | 1609-93-4 |
Synonym:None known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1609-93-4 | Cis-3-Chloroacrylic Acid | 98 | 216-545-4 |
Risk Phrases: 34
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Causes burns.Corrosive.
Potential Health Effects
Eye:
Causes eye burns. May cause chemical conjunctivitis and corneal damage.
Skin:
Causes skin burns. May cause skin rash (in milder cases), and cold and clammy skin with cyanosis or pale color.
Ingestion:
May cause severe and permanent damage to the digestive tract. Causes gastrointestinal tract burns. May cause perforation of the digestive tract. The toxicological properties of this substance have not been fully investigated. May cause systemic effects.
Inhalation:
May cause severe irritation of the respiratory tract with sore throat, coughing, shortness of breath and delayed lung edema. Causes chemical burns to the respiratory tract. The toxicological properties of this substance have not been fully investigated.
Aspiration may lead to pulmonary edema. May cause systemic effects.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Get medical aid. Do NOT allow victim to rub eyes or keep eyes closed. Extensive irrigation with water is required (at least 30 minutes).
Skin:
Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Destroy contaminated shoes.
Ingestion:
Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. Use extinguishing media appropriate to the surrounding fire. Substance is noncombustible.
Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Extinguishing Media:
Do NOT get water inside containers. For small fires, use dry chemical, carbon dioxide, or water spray. For large fires, use dry chemical, carbon dioxide, alcohol-resistant foam, or water spray.
Cool containers with flooding quantities of water until well after fire is out.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Do not breathe dust, vapor, mist, or gas. Keep container tightly closed. Avoid ingestion and inhalation. Discard contaminated shoes.
Storage:
Keep container closed when not in use. Store in a cool, dry, well-ventilated area away from incompatible substances. Corrosives area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1609-93-4: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystals
Color: white
Odor: acrid odor
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 61.00 - 62.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C3H3ClO2
Molecular Weight: 106.51
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1609-93-4 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Cis-3-Chloroacrylic Acid - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: CORROSIVE SOLID, ACIDIC, ORGANIC, N.O.S.*
Hazard Class: 8
UN Number: 3261
Packing Group: II
IMO
Shipping Name: CORROSIVE SOLID, ACIDIC, ORGANIC, N.O.S.
Hazard Class: 8
UN Number: 3261
Packing Group: II
RID/ADR
Shipping Name: CORROSIVE SOLID, ACIDIC, ORGANIC, N.O.S.
Hazard Class: 8
UN Number: 3261
Packing group: II
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: C
Risk Phrases:
R 34 Causes burns.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 1609-93-4: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1609-93-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1609-93-4 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 (E)-3-氯丙-2-烯-1-醇 (2Z)-3-chloroprop-2-en-1-ol 4643-05-4 C3H5ClO 92.5251 —— (E)-3-chloroallyl alcohol 4643-06-5 C3H5ClO 92.5251 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 反-3-氯丙烯酸 (E)-3-chloroacrylic acid 2345-61-1 C3H3ClO2 106.509 —— methyl cis-2-chloroacrylate 3510-44-9 C4H5ClO2 120.535
反应信息
-
作为反应物:描述:参考文献:名称:Glutathione Transferase Zeta-Catalyzed Biotransformation of Dichloroacetic Acid and Other α-Haloacids摘要:Dichloroacetic acid (DCA) is a common drinking-water contaminant, is hepatocarcinogenic in rats and mice, and is a therapeutic agent used clinically in the management of lactic acidosis. Recent studies show that glutathione transferase Zeta (GSTZ) catalyzes the oxygenation of DCA to glyoxylic acid [Tong et al. (1998) Biochem. J. 331, 371-374]. In the present studies, the substrate selectivity of GSTZ, the kinetics of DCA metabolism, and the fate of DCA and glutathione were investigated. The results showed that GSTZ catalyzed the oxygenation of bromochloro-, bromofluoro-, chlorofluoro-, dibromo-, and dichloroacetic acid, but not difluoroacetic acid, to glyoxylic acid. GSTZ also catalyzed the biotransformation of fluoroacetic acid to S-(carboxymethyl)glutathione, and of(R, S)-2-bromopropionic acid, (R)-, (S)-, and (R,S)-2-chloropropionic acid, and (R,S)-2-iodopropionic acid, but not (R,S)-2-fluoropropionic acid, to S-(alpha-methylcarboxymethyl)glutathione; and of 2,2-dichloropropionic acid to pyruvate. No biotransformation of 3,3-dichloropropionic acid was detected, and no GSTZ-catalyzed fluoride release from ethyl fluoroacetate and fluoroacetamide was observed. The relative rates of DCA biotransformation by hepatic cytosol were mouse > rat > human. Immunoblotting showed the presence of GSTZ in mouse, rat, and human liver cytosol. C-13 NMR spectroscopic studies showed that [2-C-13]glyoxylic acid was the only observable, stable metabolite of [2-13C]DCA. Also, glutathione was required, but was neither consumed nor oxidized to glutathione disulfide, during the oxygenation of DCA to glyoxylic acid. These results are consistent, with a reaction mechanism that involves displacement of chloride from DCA by glutathione to afford S-(alpha-chlorocarboxymethyl)glutathione, which may undergo hydrolysis to give the hemithioacetal S-(alpha-hydroxycarboxymethyl)glutathione. Elimination of glutathione from the hemithioacetal would give glyoxylic acid.DOI:10.1021/tx980144f
-
作为产物:描述:参考文献:名称:光学纯环氧聚炔的一般途径:拒食性天然产物的不对称合成摘要:描述了使用光学纯的(2R,3S)-5-溴-2,3-环氧-4-戊炔-1-醇1a作为关键中间体的通用的不对称方法。从该中间体以6个步骤进行了2种环氧聚炔天然产物的合成,总收率为42%。DOI:10.1016/s0040-4020(01)82372-3
文献信息
-
[EN] FGFR4 INHIBITORS<br/>[FR] INHIBITEURS DE FGFR4申请人:EISAI R&D MAN CO LTD公开号:WO2016164754A1公开(公告)日:2016-10-13We provide FGFR inhibitors, their salts, methods of manufacture, and methods of use.我们提供FGFR抑制剂,它们的盐,制造方法和使用方法。
-
[EN] PYRIMIDINE FGFR4 INHIBITORS<br/>[FR] INHIBITEURS DE FGFR4 PYRIMIDINE申请人:EISAI R&D MAN CO LTD公开号:WO2015057938A1公开(公告)日:2015-04-23Provided herein are compounds of Formula I useful as FGFR4 inhibitors, as well as methods of use of the same.本文提供了作为FGFR4抑制剂有用的I式化合物,以及其使用方法。
-
HYDROCHLORINATION OF 2,3-ACETYLENIC ACIDS WITH THIONYL CHLORIDE IN DIMETHYLFORMAMIDE作者:Neudo A. Urdaneta、Julio C. Herrera、José Salazar、Simón E. LópezDOI:10.1081/scc-120012990日期:2002.1ABSTRACT The reaction of 2,3-acetylenic acids (1a–d) with thionyl chloride in DMF under mild conditions gave E,Z-3-chloro-2-alkenoic acids (2a–d) or esters (3a–d) depending on treating the reaction mixture with either water or alcohols.
-
[EN] FUSED PYRIMIDINE COMPOUNDS, COMPOSITIONS AND MEDICINAL APPLICATIONS THEREOF<br/>[FR] COMPOSÉS DE PYRIMIDINE FUSIONNÉS, COMPOSITIONS ET APPLICATIONS MÉDICALES ASSOCIÉES申请人:JUBILANT BIOSYS LTD公开号:WO2021062327A1公开(公告)日:2021-04-01The present disclosure relates to a class of fused pyrimidine compounds of Formula I, their stereoisomers, tautomers, pharmaceutically acceptable salts, polymorphs, solvates, and hydrates thereof. The present disclosure also relates to a process of preparation of these fused pyrimidine compounds, and to pharmaceutical compositions containing them.
-
Irreversible inhibitors of tyrosine kinases申请人:Warner-Lambert Company公开号:US06344459B1公开(公告)日:2002-02-05The present invention provides compounds that are irreversible inhibitors of tyrosine kinases. Also provided is a method of treating cancer, restenosis, atherosclerosis, endometriosis, and psoriasis and a pharmaceutical composition that comprises a compound that is an irreversible inhibitor of tyrosine kinases.
表征谱图
-
氢谱1HNMR
-
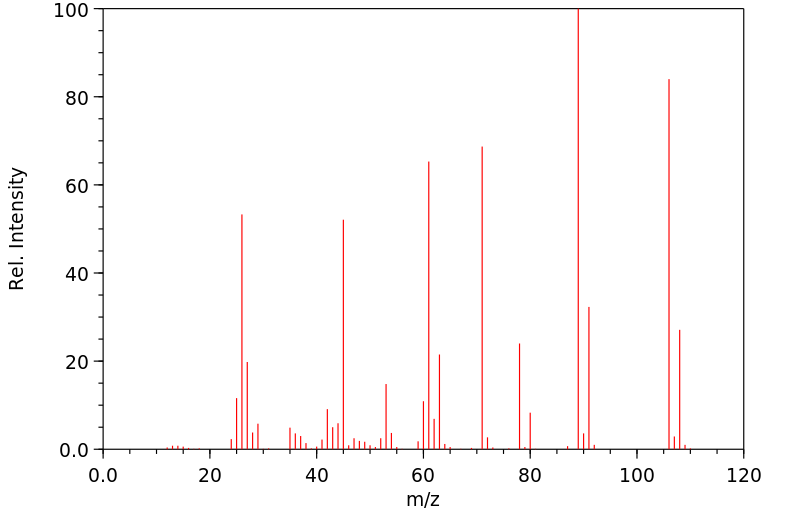
质谱MS
-
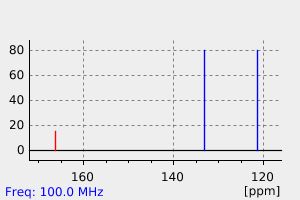
碳谱13CNMR
-
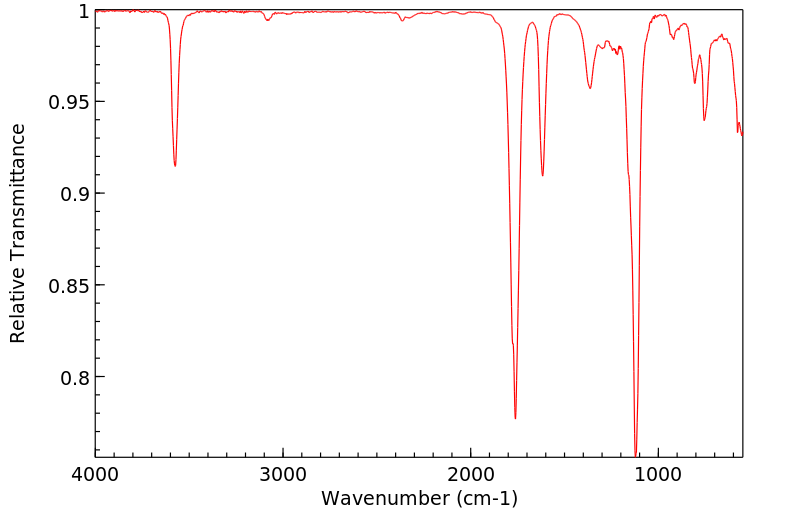
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸