假硫代乙内酰脲 | 556-90-1
中文名称
假硫代乙内酰脲
中文别名
2-氨基-1,3-噻唑啉-4-酮;2-氨基-噻唑-4-酮;噻唑;假硫内酰胺脲
英文名称
pseudothiohydantoin
英文别名
2-iminothiazolidin-4-one;2-imino-thiazolidine-4-one;2-imino-1,3-thiazolidin-4-one;2-imino-4-thiazolidinone;2-imino-1,3-thiazolan-4-one;2-Imino-1,3-thiazol-4-one
CAS
556-90-1
化学式
C3H4N2OS
mdl
MFCD00205270
分子量
116.144
InChiKey
HYMJHROUVPWYNQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:249 °C (dec.)(lit.)
-
沸点:253.5±23.0 °C(Predicted)
-
密度:1.637 g/cm3
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):-0.3
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:80.8
-
氢给体数:1
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险等级:IRRITANT
-
安全说明:S24/25
-
危险品标志:Xi
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:贮存:将密封的药瓶放入密封的主容器中,并放置在阴凉、干燥处。
SDS
| Name: | Pseudothiohydantoin 97% Material Safety Data Sheet |
| Synonym: | 4(5H)-Thiazolone, 2-Amino- |
| CAS: | 556-90-1 |
Synonym:4(5H)-Thiazolone, 2-Amino-
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 556-90-1 | Pseudothiohydantoin | 97% | 209-145-6 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 556-90-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: white to light yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 249 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C3H4N2OS
Molecular Weight: 116.14
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, nitrogen, sulfur oxides (SOx), including sulfur oxide and sulfur dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 556-90-1: XJ6276000 LD50/LC50:
Not available.
Carcinogenicity:
Pseudothiohydantoin - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 556-90-1: No information available.
Canada
CAS# 556-90-1 is listed on Canada's NDSL List.
CAS# 556-90-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 556-90-1 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
制备方法:用于有机物合成。
用途简介:
- 用于有机物合成
用途:
- 主要用于有机物的合成。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-methylamino-4-thiazolinone 16312-19-9 C4H6N2OS 130.17 —— 2-Dimethylamino-4-thiazolinone 39131-05-0 C5H8N2OS 144.197 —— 2-Hydroxymethylamino-4-thiazolinone 104959-22-0 C4H6N2O2S 146.17 N-(4-氧代-4,5-二氢噻唑-2-基)乙酰胺 2-acetamido-Δ2-thiazolin-4-one 37641-15-9 C5H6N2O2S 158.181
反应信息
-
作为反应物:参考文献:名称:(E)-5-[(1-Aryl-1 H -1,2,3-triazol-4-yl)亚甲基]噻唑烷-2,4-二酮的合成及抗癌活性摘要:摘要通过将相应的1-芳基-1 H -1,2,3-三唑-4-甲醛与噻唑烷-2,4-二酮缩合,合成了一系列新的1,2,3-三唑基噻唑烷二酮类似物。KOH。使用MTT测定法针对四种癌细胞系:A549(肺),HT-29(结肠),MCF-7(乳腺癌)和A375(黑素瘤)评估了标题化合物的体外抗癌活性。大多数化合物显示出良好的抗癌活性,但是羟基和硝基取代的衍生物显示出比其他化合物更高的活性。DOI:10.1134/s1070428020050206
-
作为产物:参考文献:名称:Kavalek, Jaromir; Said-El-Bahaie; Sterba, Vojeslav, Collection of Czechoslovak Chemical Communications, 1980, vol. 45, # 1, p. 263 - 268摘要:DOI:
-
作为试剂:描述:四氢吡咯 、 6-氯咪唑并[2,1-b][1,3]噻唑-5-甲醛 在 假硫代乙内酰脲 作用下, 生成 6-(Pyrrolidin-1-YL)imidazo[2,1-B][1,3]thiazole-5-carbaldehyde参考文献:名称:Synthesis and cardiotonic activity of imidazo[2,l-b]thiazoles bearing a lactam ring摘要:This paper describes the synthesis of 6-substituted imidazo[2,1-b] thiazoles with a lactam ring connected, by means of a methine group, to the 5-position. The pharmacological results show that interesting cardiotonic activity is obtained when the lactam ring is pseudothiohydantoin (8) or barbituric acid (9). Even the substituent at position 6 plays an important role in the pharmacological behavior of these derivatives. The following activity rank order was observed: phenyl > methyl > chlorine.DOI:10.1016/0223-5234(96)89164-1
文献信息
-
3,5-di-tertiarybutyl-4-hydroxyphenylmethylene derivatives of申请人:Warner-Lambert Company公开号:US05143928A1公开(公告)日:1992-09-01The novel 3,5-ditertiarybutyl-4-hydroxy-phenylmethylene derivative of 2-substituted thiazolidinones, oxazolidinones, and imidazolidinones as antiinflammatory agents having inhibiting activity for 5-lipoxygenase, cyclooxygenase, or both.
-
[EN] cGAS ANTAGONIST COMPOUNDS<br/>[FR] COMPOSÉS ANTAGONISTES DU CGAS申请人:IMMUNE SENSOR LLC公开号:WO2017176812A1公开(公告)日:2017-10-12Disclosed are novel compounds of Formula (I) that are cGAS antagonists, methods of preparation of the compounds, pharmaceutical compositions comprising the compounds, and their use in medical therapy.揭示了一种新型化合物的化学式(I),这些化合物是cGAS拮抗剂,涉及到这些化合物的制备方法、包含这些化合物的药物组合物,以及它们在医学治疗中的应用。
-
[EN] ARYLMETHYLIDENE HETEROCYCLES AS NOVEL ANALGESICS<br/>[FR] HÉTÉROCYCLES D'ARYLMÉTHYLIDÈNE COMME NOUVEAUX ANALGÉSIQUES申请人:CHLORION PHARMA INC公开号:WO2009097695A1公开(公告)日:2009-08-13The present invention relates to Arylmethylidene heterocycles, compositions comprising an Arylmethylidene heterocycle, and methods useful for treating or preventing pain comprising administering an effective amount of an Arylmethylidene heterocycle as depicted by the formula (Ia).The compounds, compositions, and methods of the invention are also useful for treating or preventing inflammation.
-
Anti-hepatitis-C virus activity and QSAR study of certain thiazolidinone and thiazolotriazine derivatives as potential NS5B polymerase inhibitors作者:Ghaneya S. Hassan、Hanan H. Georgey、Esraa Z. Mohammed、Farghaly A. OmarDOI:10.1016/j.ejmech.2019.111747日期:2019.12The present study reports on evaluation of anti-HCV activity and QSAR of certain arylidenethiazolidinone derivatives as potential inhibitors of HCV-NS5B polymerase. The pursued compounds involving, 5-aryliden-3-arylacetamidothiazolidin-2,4-diones 4-6(a-f), 5-arylidine-2-(N-arylacetamido)-iminothiazolidin-4-one (10) and their rigid counterparts 5-arylidinethiazolotriazines 13-15(a-f), were synthesized本研究报告了评估某些亚芳基噻唑烷酮衍生物作为 HCV-NS5B 聚合酶的潜在抑制剂的抗 HCV 活性和 QSAR。所追求的化合物包括 5-aryliden-3-arylacetamidothiazolidin-2,4-diones 4-6(af)、5-arylidine-2-(N-arylacetamido)-iminothiazolidin-4-one (10) 及其刚性对应物 5合成了 -arylidinethiazolotriazines 13-15(af),并通过光谱和元素分析确认了它们的结构。NS5B 聚合酶抑制试验的结果显示,化合物 4e 是最有效的抑制剂(IC50 = 0.035 μM),是参考药物 VCH-759(IC50 = 0.14 μM)的四倍。同时,化合物 4b、4c、5a 和 5c 以及 13b、14e 和 15c 显示出比 VCH-759 高 2 倍的活性(IC50
-
Design, synthesis and glucose uptake activity of some novel glitazones作者:Koyel Kar、Uma Krithika、Mithuna、Prabhuddha Basu、S. Santhosh Kumar、Anu Reji、B.R. Prashantha KumarDOI:10.1016/j.bioorg.2014.05.006日期:2014.10Herein, we report a library consisting of some novel glitazones containing thiazolidinedione and its bioisosteres, rhodanine and oxadiazolidine ring structures as their basic scaffold for their antidiabetic activity. Twelve novel glitazones with diverse chemical structures were designed and synthesized by adopting appropriate synthetic schemes and analyzed. Later, subjected to in vitro glucose uptake在此,我们报道了一个由一些新颖的格列酮组成的文库,这些新的格列酮含有噻唑烷二酮及其生物等排体,若丹宁和恶二唑烷环结构作为其抗糖尿病活性的基本骨架。设计并合成了十二种具有不同化学结构的格列酮类化合物,并采用适当的合成方案进行了合成和分析。之后,在大鼠不存在和存在胰岛素的情况下,进行体外葡萄糖摄取测定,以使用大鼠半隔膜确定其抗糖尿病活性。标题化合物显示葡萄糖吸收活性,范围从弱到显着。化合物4,5,9,11,15,16,19除标准药物罗格列酮外,20和20表现出相当大的葡萄糖摄取活性。化合物16恰好是该研究的候选化合物,需要进一步研究。关于它们的设计,合成,分析和葡萄糖摄取活性的插图,以及基于体外和计算机模拟研究的结构-活性关系均在此处报道。
表征谱图
-
氢谱1HNMR
-
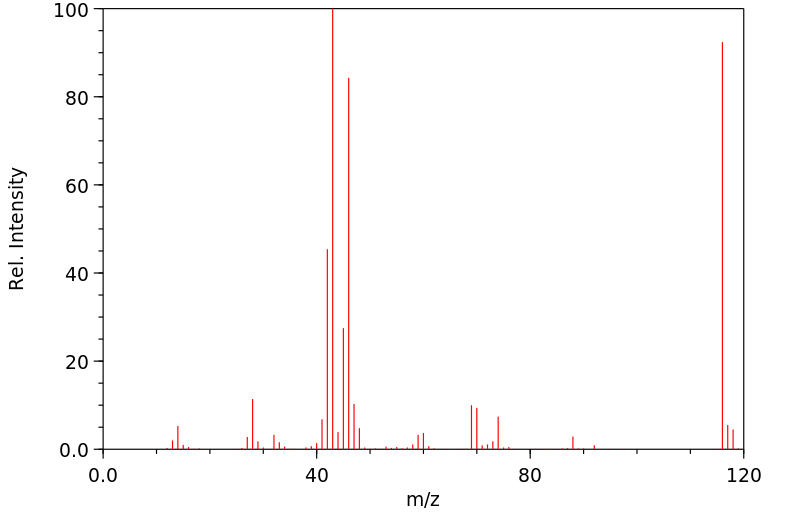
质谱MS
-
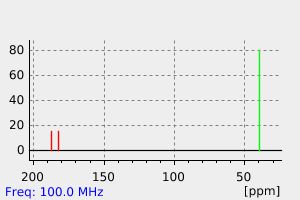
碳谱13CNMR
-
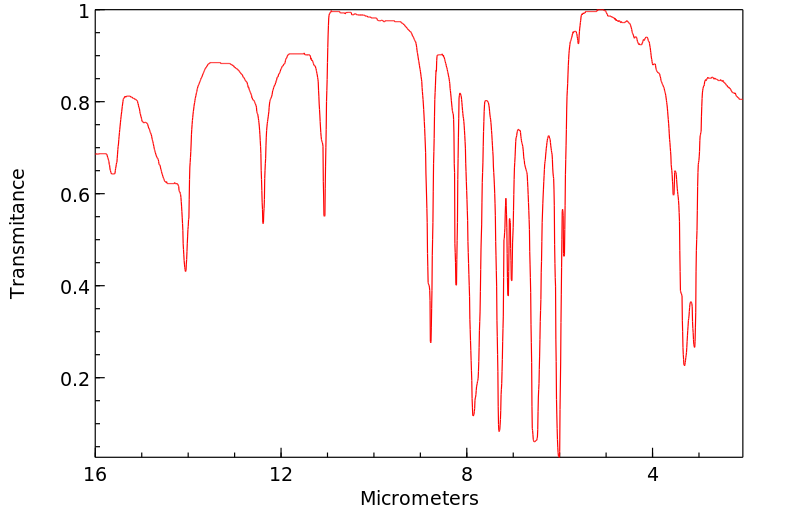
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(4S,4''S)-2,2''-环亚丙基双[4-叔丁基-4,5-二氢恶唑]
香豆素-6-羧酸
顺式-3a,5,6,6a-四氢-3-(1-甲基乙基)-4H-环戊二烯并[d]异恶唑
锌离子载体IV
钐(III) 离子载体 II
苯,1-(2E)-2-丁烯-1-基-2-氟-
苯,(2,2-二氟乙烯基)-
聚二硫二噻唑烷
缩胆囊肽9
绕丹酸钠
盐(1:?)5'-尿苷酸,钠
甲酰乙内脲
甲巯咪唑
甲基羟甲基油基噁唑啉
甲基5-羟基-3,5-二甲基-4,5-二氢-1H-吡唑-1-羧酸酯
甲基5-甲基-4,5-二氢-1H-吡唑-3-羧酸酯
甲基5-甲基-4,5-二氢-1H-吡唑-1-羧酸酯
甲基5-氰基-4,5-二氢-1,2-恶唑-3-羧酸酯
甲基5-乙炔基-4,5-二氢-1H-吡唑-3-羧酸酯
甲基5-(羟基甲基)-4,5-二氢-1,2-恶唑-3-羧酸酯
甲基4-甲基-5-氧代-4,5-二氢-1H-吡唑-3-羧酸酯
甲基4-甲基-4,5-二氢-1H-吡唑-3-羧酸酯
甲基4-乙炔基-4,5-二氢-1H-吡唑-3-羧酸酯
甲基4,5-二氮杂螺[2.4]庚-5-烯-6-羧酸酯
甲基4,5-二氢-5-乙基-1H-吡唑-1-羧酸酯
甲基3-甲基-4,5-二氢-1,2-恶唑-4-羧酸酯
甲基(E)-3-[6-[1-羟基-1-(4-甲基苯基)-3-(1-吡咯烷基)丙基]-2-吡啶基]丙烯酰酸酯
甲基(5-氧代-4,5-二氢-1,2-恶唑-3-基)乙酸酯
环戊二烯并[d]咪唑-2,5(1H,3H)-二硫酮
环己羧酸,3-氨基-2-甲氧基-,甲基酯,(1S,2S,3S)-
溶剂黄93
溴化1-十六烷基-3-甲基咪唑
溴化1-十二烷基-2,3-二甲基咪唑
泰比培南酯中间体
泰比培南酯中间体
氨甲酸,[4,5-二氢-4-(碘甲基)-2-噻唑基]-,1,1-二甲基乙基酯(9CI)
氨基甲硫酸,[2-[[(2-羰基-1-咪唑烷基)硫代甲基]氨基]乙基]-,O-甲基酯
异噻唑,4,5-二氯-2,5-二氢-2-辛基-
希诺米啉
四氟硼酸二氢1,3-二(叔-丁基)-4,5--1H-咪唑正离子
四唑硝基紫
噻唑烷-2,4-二酮-2-缩氨基脲
噻唑丁炎酮
噻唑,4,5-二氢-4-(1-甲基乙基)-,(S)-
噁唑,4,5-二氢-4,4-二甲基-2-(5-甲基-2-呋喃基)-
噁唑,2-庚基-4,5-二氢-
咪唑烷基脲
吡嗪,2,3-二氢-5,6-二甲基-2-丙基-
叔-丁基3-羟基-1,4,6,7-四氢吡唑并[4,3-c]吡啶-5-羧酸酯
双吡唑啉酮