2-氨基-3-疏基丙酸甲酯盐酸盐 | 5714-80-7
中文名称
2-氨基-3-疏基丙酸甲酯盐酸盐
中文别名
DL-半胱氨酸甲酯盐酸盐
英文名称
cysteine methyl ester hydrochloride
英文别名
dl-cysteine methyl ester hydrochloride;Cysteinmethylesterhydrochlorid;Hydron;methyl 2-amino-3-sulfanylpropanoate;chloride;hydron;methyl 2-amino-3-sulfanylpropanoate;chloride
CAS
5714-80-7
化学式
C4H10NO2S*Cl
mdl
MFCD00173915
分子量
171.648
InChiKey
WHOHXJZQBJXAKL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:175-182 °C
计算性质
-
辛醇/水分配系数(LogP):-0.38
-
重原子数:9
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:53.3
-
氢给体数:3
-
氢受体数:4
安全信息
-
海关编码:2930909090
-
储存条件:室温
SDS
反应信息
-
作为反应物:描述:4-(2-hydroxy-4-methylphenoxy)benzaldehyde 、 2-氨基-3-疏基丙酸甲酯盐酸盐 在 三乙胺 作用下, 以 二氯甲烷 为溶剂, 以56%的产率得到2-[4-(2-hydroxy-4-methylphenoxy)phenyl]thiazolidine-4-carboxylic acid methyl ester参考文献:名称:WO2006/71471摘要:公开号:
-
作为产物:描述:参考文献:名称:[EN] THIAZOLIDINONE AMIDES, THIAZOLIDINE CARBOXYLIC ACID AMIDES, METHODS OF MAKING, AND USES THEREOF
[FR] AMIDES DE THIAZOLIDINONE, AMIDES D'ACIDE CARBOXYLIQUE DE THIAZOLIDINE, PROCEDES DE FABRICATION ET UTILISATIONS CORRESPONDANTS摘要:根据公式(I)和(II)披露了替代噻唑烷酮羧酸酰胺和替代噻唑烷羧酸酰胺,其中各种取代基如规范中所定义。还披露了制备这些化合物的方法,含有这些化合物的药物组合物,以及它们的用途,特别是用于治疗或预防癌症。公开号:WO2005049591A1 -
作为试剂:参考文献:名称:Tetrahedron Lett 2002, 43, 6561-6562摘要:DOI:
文献信息
-
[EN] 2-(1H-INDOLE-3-CARBONYL)-THIAZOLE-4-CARBOXAMIDE DERIVATIVES AND RELATED COMPOUNDS AS ARYL HYDROCARBON RECEPTOR (AHR) AGONISTS FOR THE TREATMENT OF E.G. ANGIOGENESIS IMPLICATED OR INFLAMMATORY DISORDERS<br/>[FR] DÉRIVÉS DE 2-(1H-INDOLE-3-CARBONYL)-THIAZOLE-4-CARBOXAMIDE ET COMPOSÉS CORRESPONDANTS UTILISÉS EN TANQUE QUE AGONISTES DU RÉCEPTEUR D'HYDROCARBURE ARYLE (AHR) UTILISÉS POUR LE TRAITEMENT DE, P.EX., DE L'ANGIOGENÈSE IMPLIQUÉE OU DE TROUBLES INFLAMMATOIRES申请人:IKENA ONCOLOGY INC公开号:WO2021127302A1公开(公告)日:2021-06-242-(1H-lndole-3-carbonyl)-thiazole-4-carboxamide derivatives and the corresponding imidazole, oxazole and thiophene derivatives and related compounds as aryl hydrocarbon receptor (AHR) agonists for the treatment of angiogenesis implicated disorders, such as e.g. retinopathy, psoriasis, rheumatoid arthritis, obesity and cancer, or inflammatory disorders. The present description discloses the synthesis and characterisation of exemplary compounds as well as pharmacological data thereof (e.g. pages 27 to 32 and 59 to 219; examples 1 to 8; compounds 1-1 to 1-97; tables 1-a, 2 and 3).
-
Synthesis of New 3,4-Disubstituted 2,5-Dihydro-1<i>H</i>-pyrrol-1-yloxyl Spin-Label Reagents via Allylic Rearrangements作者:Kálmán Hideg、Cecília P. Sár、Olga H. Hankovszky、Tünde Tamás、Gyula JerkovichDOI:10.1055/s-1993-25870日期:——Several 3,4-disubstituted 2,5-dihydro-1H-pyrrol-1-yloxyl radicals were synthesized. 2,5-Dihydro-3-hydroxymethyl-2,2,5,5-tetramethyl-1H-pyrrol-1-yloxyl radical (1) was reacted with triethyl orthoacetate in a Claisen rearrangement to give the exoolefinic compound 2. This was converted to its 1-acetoxyl derivative 3, which was then brominated in the allylic position. Subsequent rearrangement gave the endoolefinic compound 1-acetoxyl-4-bromomethyl-3-ethoxycarbonylmethyl-2,2,5,5-tetramethyl-1H-pyrrole radical (4). The allylic bromine could be replaced with nucleophiles (NaSCN, KSeCN, thiourea, selenourea, NaN3 and KSSO2Me) to give 5a-e and 26. The diamagnetic thiocyanates and selenocyanates could be reduced with sodium borohydride to the free thiol and selenol monoradicals, which were oxidized to reversible, ester-functionalized disulfide or diselenide diradical reagents 24 and 25.合成了几个3,4-二取代的2,5-二氢-1H-吡咯-1-氧基自由基。2,5-二氢-3-羟甲基-2,2,5,5-四甲基-1H-吡咯-1-氧基自由基(1)经Claisen重排反应与三乙基原乙酸酯反应,得到外烯烃化合物2。将其转化为1-乙酸氧基衍生物3,然后在烯丙位进行溴化。随后的重排反应得到内烯烃化合物1-乙酸氧基-4-溴甲基-3-乙氧羰甲基-2,2,5,5-四甲基-1H-吡咯自由基(4)。烯丙位的溴可以被亲核试剂(NaSCN、KSeCN、硫脲、硒脲、NaN3和KSSO2Me)取代,得到5a-e和26。无磁性的硫氰酸盐和硒氰酸盐可以用硼氢化钠还原成自由的硫醇和硒醇单自由基,这些自由基被氧化成可逆的、酯功能化的二硫化物或二硒化物双自由基试剂24和25。
-
Synthesis of the molecular hybrid inspired by Largazole and Psammaplin A作者:Xiaoling Yu、Bingbing Zhang、Guangsheng Shan、Yue Wu、Feng-Ling Yang、Xinsheng LeiDOI:10.1016/j.tet.2017.12.025日期:2018.2distinguishing examples, Largazole and Psammaplin A, which possess macrocyclic depsipeptide and simple linear amide scaffold respectively, we designed one novel molecular hybrid by replacing the alkene moiety in Largazole with a semirigid amide bond. This hybrid compound has been synthesized from l-malic acid in 10 steps with an overall yield of 7%. The preliminary biological assays suggest that the replacement
-
Bisamide bisthiol compounds useful for making technetium radiodiagnostic申请人:The Children's Medical Center Corporation公开号:US04673562A1公开(公告)日:1987-06-16A radiodiagnostic bisamido-bisthio ligand useful for producing Tc-labelled radiodiagnostic renal agents is described. The ligand forms a complex with the radionuclide .sup.99m Tc suitable for administration as a radiopharmaceutical to obtain images of the kidney for diagnosis of kidney disfunction.
-
ARYL HYDROCARBON RECEPTOR MODULATOR申请人:Ariagen, Inc.公开号:US20190307731A1公开(公告)日:2019-10-10Disclosed is an aryl hydrocarbon receptor modulator of formula (I), and a pharmaceutically acceptable salt thereof, wherein R′ is H, CN, CH 2 (OH)R 0 , C m H 2m+1 , C n H 2n-1 , C n H 2n-3 , two R a are independently H or two R a together form ═O or ═N—W 3 —R 1 ; A is a C 6 to C 10 aromatic ring unsubstituted or substituted with 1 to 3 R, or a C 2 -C 10 heteroaromatic ring interrupted by 1 to 5 heteroatoms selected from N, O, and S or a 4 to 7 membered nonaromatic heterocyclic ring containing C═N and interrupted by 1 to 3 heteroatoms selected from N, O, and S, with either one unsubstituted or substituted with 1 to 3 R; Q is R, or is a C 6 to C 10 aromatic ring or a C 2 to C 10 heteroaromatic ring unsubstituted or substituted with 1 to 3 R and interrupted by 1 to 5 heteroatoms selected from N, O, and S; and R is R c which is C-attached or R N which is N-attached. The compounds of formula (I) of the present invention can regulate AhR activity, and can be used to inhibit the growth of cancer cells and inhibit the metastasis and invasion of tumor cells.本发明揭示了一种化学式(I)的芳香烃受体调节剂及其药用可接受盐,其中R′为H、CN、CH2(OH)R0、CmH2m+1、CnH2n-1、CnH2n-3,两个Ra独立地为H或两个Ra共同形成═O或═N—W3—R1;A为未取代或取代1至3个R的C6至C10芳香环,或由N、O和S中选择的1至5个杂原子中断的C2-C10杂芳环,或含有C═N并由N、O和S中选择的1至3个杂原子中断的4至7个成员的非芳杂环,其中一个未取代或取代1至3个R;Q为R,或者是未取代或取代1至3个R并由N、O和S中断的C6至C10芳香环或C2至C10杂芳环;R为C-连接的Rc或N-连接的RN。本发明的化合物可以调节AhR活性,可用于抑制癌细胞的生长,以及抑制肿瘤细胞的转移和侵袭。
表征谱图
-
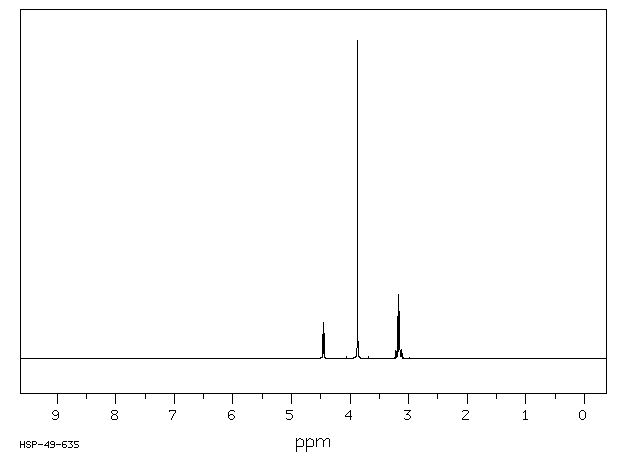
氢谱1HNMR
-
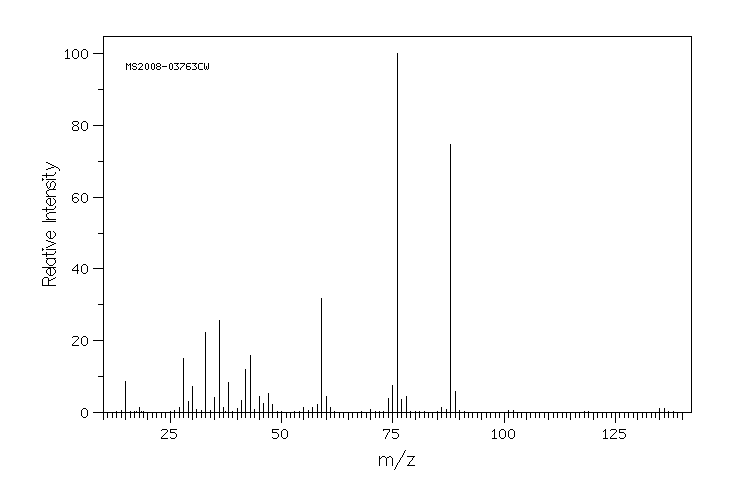
质谱MS
-
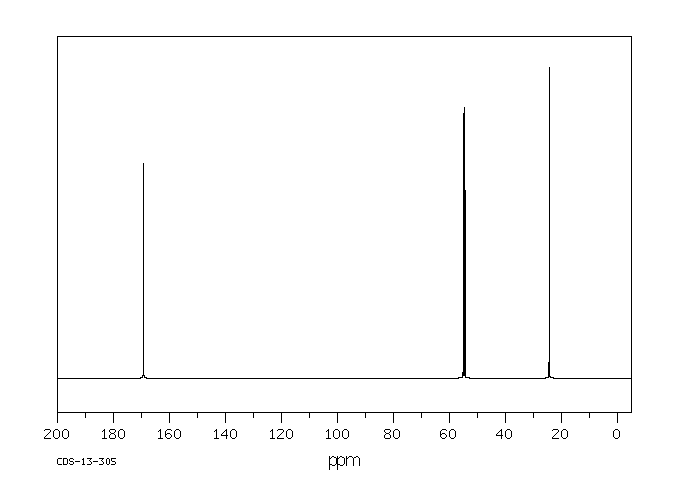
碳谱13CNMR
-
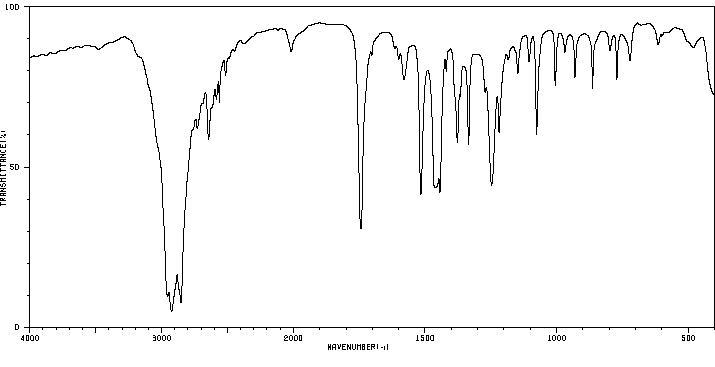
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸