N,2-二苯基丙酰胺 | 19341-03-8
中文名称
N,2-二苯基丙酰胺
中文别名
——
英文名称
N-phenyl-2-phenylpropanamide
英文别名
N,2-diphenylpropanamide
CAS
19341-03-8
化学式
C15H15NO
mdl
——
分子量
225.29
InChiKey
PIZLNJQSSMQFOE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:133-134 °C
-
沸点:417.9±24.0 °C(Predicted)
-
密度:1.123±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:17
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.133
-
拓扑面积:29.1
-
氢给体数:1
-
氢受体数:1
SDS
反应信息
-
作为反应物:描述:参考文献:名称:通过C的直接偶联合成羟吲哚。H和C ?H中心摘要:sp 2 / sp 3 在一起:可以通过芳基CH和CH中心的直接分子内氧化偶合,使用新颖有效的方法合成3,3-二取代的羟吲哚(参见方案; DMF = N,N-二甲基甲酰胺)。DOI:10.1002/anie.200805652
-
作为产物:描述:参考文献:名称:在不存在碱的情况下,使用亚硫酰氯在二甲基乙酰胺中直接由羧酸形成丙烯腈,丙烯酰胺和酰胺摘要:描述了一种通用的一锅法,该方法可在亚甲基乙酰胺(DMAC)中用亚硫酰氯和化学计算量的苯胺依次处理该酸时,将丙烯酸迅速转化为苯胺,产率为88-98%,而DMAC提供了速率和稳定性方面的优势。使用DMF。在形成酸酐的过程中,DMAC的使用扩展到了其他有机酸。苄胺酰胺也可以使用化学计量的苄胺形成,并在不存在其他碱的情况下通过加热使其完全。另外,显示了在20℃下添加过量的叔丁胺可以容易地形成叔丁酰胺。DOI:10.1021/op060125+
文献信息
-
Asymmetric Markovnikov Hydroaminocarbonylation of Alkenes Enabled by Palladium-Monodentate Phosphoramidite Catalysis作者:Ya-Hong Yao、Hui-Yi Yang、Ming Chen、Fei Wu、Xing-Xing Xu、Zheng-Hui GuanDOI:10.1021/jacs.0c11249日期:2021.1.13n of alkenes with anilines has been developed for the atom-economical synthesis of 2-substituted propanamides bearing an α-stereocenter. A novel phosphoramidite ligand L16 was discovered which exhibited very high reactivity and selectivity in the reaction. This asymmetric Markovnikov hydroaminocarbonylation employs readily available starting materials and tolerates a wide range of functional groups
-
Copper-catalyzed transformation of alkyl nitriles to <i>N</i>-arylacetamide using diaryliodonium salts作者:Romain Sallio、Pierre-Adrien Payard、Paweł Pakulski、Iryna Diachenko、Indira Fabre、Sabine Berteina-Raboin、Cyril Colas、Ilaria Ciofini、Laurence Grimaud、Isabelle GillaizeauDOI:10.1039/d1ra02305e日期:——This work reports a simple and efficient method for the copper-catalyzed redox-neutral transformation of alkyl nitriles using eco-friendly diaryliodonium salts and leading to N-arylacetamides. The method features high efficiency, broad substrate scope and good functional group tolerance.
-
Palladium-Catalyzed Hydrocarbonylative C–N Coupling of Alkenes with Amides作者:Xibing Zhou、Guoying Zhang、Bao Gao、Hanmin HuangDOI:10.1021/acs.orglett.8b00538日期:2018.4.20An efficient palladium-catalyzed hydrocarbonylative C–N coupling of alkenes with amides has been developed. The reaction was performed via hydrocarbonylation of alkenes, followed by acyl metathesis with amides. Both intermolecular and intramolecular reactions proceed smoothly to give either branched or linear amides in high turnover number (3500) with NH4Cl or NMP·HCl as a proton source under the palladium
-
Clean, reusable and low cost heterogeneous catalyst for amide synthesis作者:James W. Comerford、James H. Clark、Duncan J. Macquarrie、Simon W. BreedenDOI:10.1039/b901581g日期:——We have developed a heterogeneous silica catalyst that can effectively catalyse amide synthesis from acid and amine, without production of toxic by-products and with the advantage of being readily available, low cost, environmentally benign and reusable.我们已经开发出一种异相二氧化硅催化剂,能够有效地催化酸和胺合成酰胺,过程中不产生有毒副产品,并且具有易得、低成本、环境友好及可重复使用的优点。
-
Catalytic α-Hydroarylation of Acrylates and Acrylamides via an Interrupted Hydrodehalogenation Reaction作者:Alena M. Vasquez、John A. Gurak、Candice L. Joe、Emily C. Cherney、Keary M. EngleDOI:10.1021/jacs.0c03040日期:2020.6.10The palladium-catalyzed, α-selective hydroarylation of acrylates and acrylamides is reported. Under optimized conditions, this method is highly tolerant of a wide range of substrates including those with base sensitive functional groups and/or multiple enolizable carbonyl groups. A detailed mechanistic study was undertaken, and the high selectivity of this transformation was shown to be enabled by
表征谱图
-
氢谱1HNMR
-
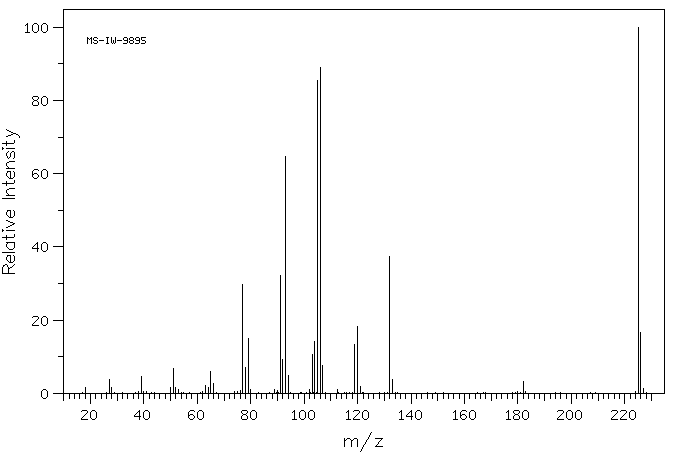
质谱MS
-
碳谱13CNMR
-
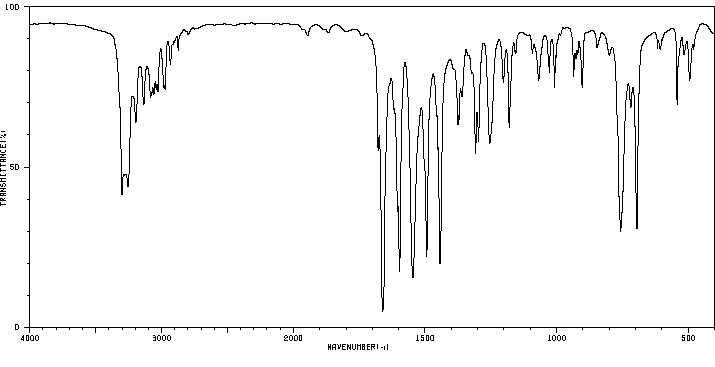
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫