N,2-二苯基喹唑啉-4-胺 | 40288-70-8
中文名称
N,2-二苯基喹唑啉-4-胺
中文别名
——
英文名称
2-phenyl-4-phenylaminoquinazoline
英文别名
N,2-diphenylquinazolin-4-amine
CAS
40288-70-8
化学式
C20H15N3
mdl
MFCD02042764
分子量
297.359
InChiKey
CMVDRFFMSYMSRT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:220-222 °C(Solv: hexane (110-54-3); ethyl acetate (141-78-6))
-
沸点:402.7±27.0 °C(Predicted)
-
密度:1.232±0.06 g/cm3(Predicted)
-
溶解度:2.5 [ug/mL]
计算性质
-
辛醇/水分配系数(LogP):5.4
-
重原子数:23
-
可旋转键数:3
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:37.8
-
氢给体数:1
-
氢受体数:3
SDS
上下游信息
反应信息
-
作为反应物:描述:N,2-二苯基喹唑啉-4-胺 在 碘苯二乙酸 作用下, 反应 0.5h, 以98%的产率得到6-苯基-苯并咪唑并[1,2- C]喹啉参考文献:名称:Rhodium(III)-catalyzed [4 + 2] annulation of N-arylbenzamidines with 1,4,2-dioxazol-5-ones: Easy access to 4-aminoquinazolines via highly selective C H bond activation摘要:DOI:10.1016/j.cclet.2021.02.061
-
作为产物:描述:参考文献:名称:发现新型喹唑啉衍生物作为强效抗肿瘤剂摘要:在这项工作中,我们设计并合成了一系列新型喹唑啉衍生物 6-19,然后分别评估了它们对 MGC-803、MCF-7、PC-9、A549 和 H1975 的广谱抗肿瘤活性。它们中的大多数对五种测试的细胞系表现出低微摩尔细胞毒性。特别是,化合物18对 MGC-803 细胞表现出纳摩尔水平的抑制活性,IC 50值为 0.85 μM,表明对 GES-1 的选择性约为 32 倍(IC 50 = 26.75 μM)。进一步的临床前评估表明,化合物18显着抑制 MGC-803 细胞的迁移,诱导 G2/M 细胞周期停滞,诱导 MGC-803 凋亡,导致 Bcl-2 和 Mcl-1 的表达降低,上调 Bax 的表达和裂解的 PARP。急性毒性试验未观察到小鼠死亡或明显的病理损伤。体内抗肿瘤评估表明,化合物18显着降低了平均肿瘤体积和肿瘤重量,对体重没有任何影响,优于5-Fu。因此,化合物18可作为未来进一步开发抗肿瘤药物的先导化合物。DOI:10.3390/molecules27123906
文献信息
-
Synthesis, 2D-QSAR Studies and Biological Evaluation of Quinazoline Derivatives as Potent Anti-Trypanosoma cruzi Agents作者:Mariela Bollini、Ana M. Bruno、María E. Niño、Juan J. Casal、Leandro D. Sasiambarrena、Damián A.G. Valdez、Leandro Battini、Vanesa R. Puente、María E. LombardoDOI:10.2174/1573406414666181005145042日期:2019.4.12disease. Therefore, continuous research is an urgent need so as to discover novel therapeutic alternatives. Objective: The development of safer and more efficient therapeutic anti-T. cruzi drugs continues to be a major goal in trypanocidal chemotherapy. Method: Synthesis, 2D-QSAR and drug-like physicochemical properties of a set of quinazolinone and quinazoline derivatives were studied as trypanocidal背景:恰加斯病感染了全世界约700万人。目前只有两种药物可用于治疗这种寄生虫病,即苯并尼达唑(Bzn)和硝呋替莫(Nfx)。两种药物在该疾病的慢性期均具有有限的疗效。因此,持续的研究是迫切需要以发现新颖的治疗选择。 目的:开发更安全,更有效的治疗性抗-T。Cruzi药物仍然是锥虫杀伤性化疗的主要目标。 方法:研究了一组喹唑啉酮和喹唑啉衍生物作为锥虫杀螨剂的合成,2D-QSAR和类药物理化性质。体外针对克氏锥虫(图拉胡恩(Tulahuen)菌株,Tul 2原种)的表鞭毛纲动物和血流类锥鞭毛纲动物筛选了所有化合物。 结果:在合成和测试的34种化合物中,有6种化合物(5a,5b,9b,9h,13f和13p)对前鞭毛体和三鞭毛体均显示出显着活性,而对Vero细胞无毒性。 结论:这些喹唑啉酮和喹唑啉衍生物的抗原生动物活性代表了针对旨在开发查加斯病的新型化学疗法的药物化学程序的有趣起点。
-
Synthesis of 4-Arylaminoquinazolines and 2-Aryl-4-arylaminoquinazolines from 2-Aminobenzonitrile, Anilines and Formic Acid or Benzaldehydes作者:Wojciech Szczepankiewicz、Jerzy Suwiński、Robert BujokDOI:10.1016/s0040-4020(00)00899-1日期:2000.11gave respective 2-amino-N-arylbenzamidines. 4-Arylaminoquinazolines lacking a substituent at the 2 position were obtained directly by heating 2-amino-N-arylbenzamidines in formic acid; in similar conditions other carboxylic acids did not react with the amidines. The latter when treated with aldehydes afforded 2-aryl-4-arylimino-1H-2,3-dihydroquinazolines readily oxidizable by potassium permanganate to
-
THERAPEUTIC COMPOUNDS AND RELATED METHODS OF USE申请人:Suzuki Masaki公开号:US20150307477A1公开(公告)日:2015-10-29Methods of treating disorders using compounds that modulate striatal-enriched tyrosine phosphatase (STEP) are described herein. Exemplary disorders include schizophrenia and cognitive deficit.
-
Synthesis of quinazolinones from anthranilamides and aldehydes via metal-free aerobic oxidation in DMSO作者:Na Yeun Kim、Cheol-Hong CheonDOI:10.1016/j.tetlet.2014.02.065日期:2014.4A highly environmentally benign protocol for the synthesis of quinazolinones from anthranilamides and aldehydes via aerobic oxidation was developed in wet DMSO. This protocol is operationally simple, exhibits broad substrate scope, and does not need toxic metal catalysts and bases. In addition, the utility of this transformation was further demonstrated by converting the resulting quinazolinones into
-
디메틸설폭사이드 용매하에서 호기성 산화법을 이용한 퀴나졸리논 유도체의 제조방법申请人:Korea University Research and Business Foundation 고려대학교 산학협력단(220040170680) BRN ▼209-82-08298公开号:KR101580821B1公开(公告)日:2015-12-30본 발명은 금속과 염기가 배제된 디메틸설폭사이드(dimethylsulfoxid, DMSO) 용매하에서 산소원를 산화제로 사용하는 호기성 산화법을 이용하여 퀴나졸리논 유도체를 제조하는 방법에 관한 것으로, 본 발명에 따른 퀴나졸리논 유도체의 제조방법은 팔라듐이나 이리듐 등과 같은 금속촉매를 필요로 하지 않아 잔존하는 금속에 의한 독성문제가 없으며, 강한 산 또는 염기조건, 저온 반응 및 무수조건의 반응과 같은 까다로운 공정을 필요로 하지 않으며, 별도의 정제공정을 필요로 하지 않아 경제적이고, 간단하게 안트라닐아미드 유도체와 알데히드원을 반응시켜 퀴나졸리논 유도체를 도입할 수 있다.
表征谱图
-
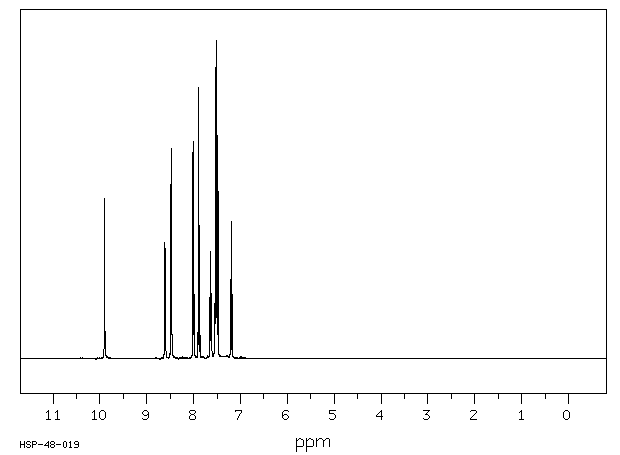
氢谱1HNMR
-
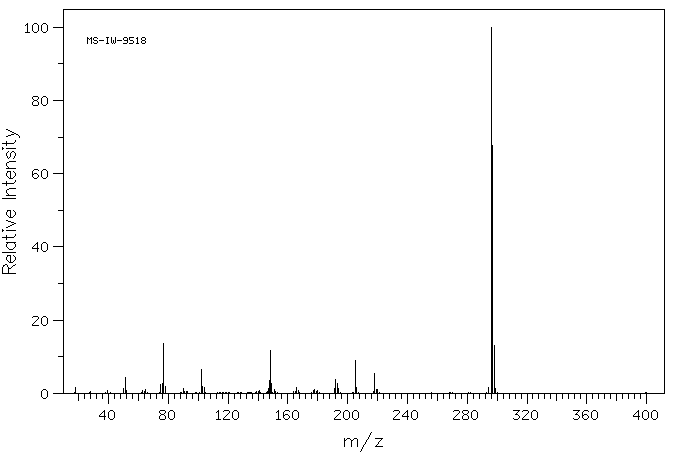
质谱MS
-
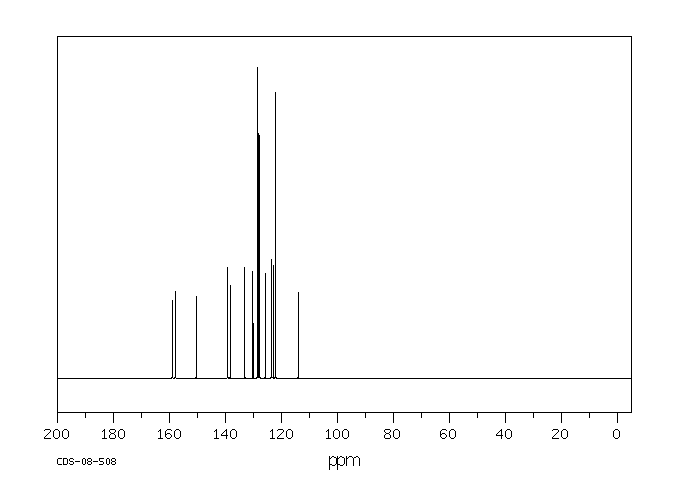
碳谱13CNMR
-
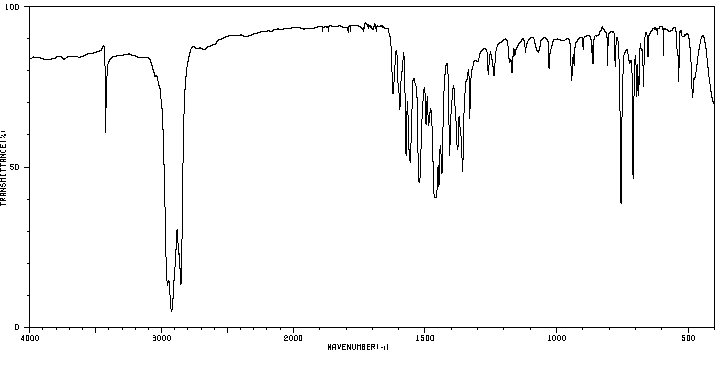
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(12羟基吲[2,1-b〕喹唑啉-6(12H)-酮)
黑暗猝灭剂BHQ-3,BHQ-3NHS
鸭嘴花酚碱
鸭嘴花碱酮;(S)-2,3-二氢-3,7-二羟基吡咯并[2,1-b]喹唑啉-9(1H)-酮
鸭嘴花碱酮
鸭嘴花碱盐酸盐
鲁米诺单钠盐
鲁米诺
骆驼蓬碱
颜料蓝64
颜料蓝60
顺式-卤夫酮
顺式-(喹喔啉-2-基)丙烯腈1,4-二氧化物
非奈利酮
青黛酮
雷替曲塞杂质1
阿法替尼杂质J
阿法替尼杂质I
阿法替尼杂质28
阿法替尼杂质18
阿法替尼杂质13
阿法替尼杂质
阿法替尼中间体
阿法替尼
阿法替尼
阿朴藏红
阿巴康唑
阿夫唑嗪杂质A
阿夫唑嗪杂质
阿夫唑嗪EP杂质C
阿夫唑嗪
阿喹司特
阿呋唑嗪杂质
阿呋唑嗪杂质
铜迈星
铁诱导细胞死亡激活剂
钠四丙基硼酸酯
酸性蓝98
酸性红101
酮色林醇
酞联氮基[2,3-b]酞嗪-5,14-二酮,7,12-二氢-
酞嗪-5-羧酸
酞嗪-2-氧化物
酚藏花红
酚嗪
酒石酸溴莫尼定
邻苯二甲酰肼
还原黄6GD
还原蓝6
达尼喹酮