甲磺酸丙酯 | 1912-31-8
中文名称
甲磺酸丙酯
中文别名
甲磺酸正丙酯
英文名称
propyl methanesulfonate
英文别名
n-propyl methanesulfonate;n-propyl methane sulphonate
CAS
1912-31-8
化学式
C4H10O3S
mdl
——
分子量
138.188
InChiKey
DKORSYDQYFVQNS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:110°C/20mmHg(lit.)
-
密度:1.132 (estimate)
-
溶解度:氯仿(微溶)、甲醇(微溶)
计算性质
-
辛醇/水分配系数(LogP):0.5
-
重原子数:8
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:51.8
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2905199090
-
危险性防范说明:P264,P270,P301+P312+P330,P501
-
危险性描述:H302
-
储存条件:室温
SDS
Section I.Chemical Product and Company Identification
Chemical Name Propyl Methanesulfonate
Portland OR
Synonym Methanesulfonic acid, propyl ester (CA INDEX NAME);
Methanesulfonic Acid Propyl Ester
Chemical Formula C4H10O3S
CAS Number 1912-31-8
Section II. Composition and Information on Ingredients
Toxicology Data
Chemical Name CAS Number Percent (%) TLV/PEL
1912-31-8 Min. 98.0 (GC) Not available. Not available.
Propyl Methanesulfonate
Section III. Hazards Identification
Acute Health Effects Harmful if ingested or inhaled. Minimize exposure to this material. Severe overexposure can result in injury or death.
Follow safe industrial hygiene practices and always wear proper protective equipment when handling this compound.
Chronic Health Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Reproductive effects.
Rat TDLo Intraperitoneal 400 mg/kg, male 1 day prior to mating
Toxic Effects:
Effects on Fertility - Male fertility index
Mouse TDLo Intraperitoneal 600 mg/kg, female 1 day prior to mating
Toxic Effects:
Effects on Fertility - Litter size
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Section IV. First Aid Measures
Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15
Eye Contact
minutes. Get medical attention.
In case of contact, immediately flush skin with plenty of water. Remove contaminated clothing and shoes. Wash clothing
Skin Contact
before reuse. Thoroughly clean shoes before reuse. Get medical attention.
Inhalation If the victim is not breathing, perform mouth-to-mouth resuscitation. Loosen tight clothing such as a collar, tie, belt or
waistband. If breathing is difficult, oxygen can be administered. Seek medical attention if respiration problems do not
improve.
Ingestion INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat.
Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing, perform mouth-to-mouth
resuscitation. Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the toxic
material was ingested; the absence of such signs, however, is not conclusive.
Section V. Fire and Explosion Data
Not available.
May be combustible at high temperature. Auto-Ignition
Flammability
Flammable Limits Not available.
Flash Points Not available.
Combustion Products These products are toxic carbon oxides (CO, CO2), sulfur oxides (SOx).
Fire Hazards
Not available.
Risks of explosion of the product in presence of mechanical impact: Not available.
Explosion Hazards
Risks of explosion of the product in presence of static discharge: Not available.
Fire Fighting Media
SMALL FIRE: Use DRY chemical powder.
LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
and Instructions
Consult with local fire authorities before attempting large scale fire-fighting operations.
Continued on Next Page
Propyl Methanesulfonate
Section VI. Accidental Release Measures
Spill Cleanup Harmful material. Possibly mutagenic material.
Absorb with an inert material and put the spilled material in an appropriate waste disposal. Consult federal, state, and/or local
Instructions
authorities for assistance on disposal.
Section VII. Handling and Storage
Handling and Storage HARMFUL. POSSIBLE MUTAGEN. Keep away from heat. Mechanical exhaust required. When not in use, tightly seal the
container and store in a dry, cool place. Avoid excessive heat and light. Do not breathe gas/fumes/ vapor/spray.
Information
Always store away from incompatible compounds such as oxidizing agents.
Section VIII. Exposure Controls/Personal Protection
Provide exhaust ventilation or other engineering controls to keep the airborne concentrations of vapors below their respective
Engineering Controls
threshold limit value. Ensure that eyewash station and safety shower is proximal to the work-station location.
Splash goggles. Lab coat. Vapor respirator. Boots. Gloves. Suggested protective clothing might not be sufficient; consult a
Personal Protection
specialist BEFORE handling this product. Be sure to use a MSHA/NIOSH approved respirator or equivalent.
Exposure Limits Not available.
Section IX. Physical and Chemical Properties
Liquid. (Clear, colorless ~ light yellow.) Solubility
Physical state @ 20°C Not available.
1.15 (water=1)
Specific Gravity
138.19
Molecular Weight Partition Coefficient Not available.
Boiling Point 110°C (230°F) @ 20 mmHg Vapor Pressure Not available.
Not available. Not available.
Melting Point Vapor Density
1.42 Volatility Not available.
Refractive Index
Not available.
Critical Temperature Not available. Odor
Viscosity Not available. Taste Not available.
Section X. Stability and Reactivity Data
Stability
This material is stable if stored under proper conditions. (See Section VII for instructions)
Conditions of Instability Avoid excessive heat and light.
Incompatibilities
Reactive with oxidizing agents.
Section XI. Toxicological Information
PB2680000
RTECS Number
Routes of Exposure Eye Contact. Ingestion. Inhalation.
Not available.
Toxicity Data
Chronic Toxic Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Reproductive effects.
Rat TDLo Intraperitoneal 400 mg/kg, male 1 day prior to mating
Toxic Effects:
Effects on Fertility - Male fertility index
Mouse TDLo Intraperitoneal 600 mg/kg, female 1 day prior to mating
Toxic Effects:
Effects on Fertility - Litter size
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Harmful if ingested or inhaled. Minimize exposure to this material. Severe overexposure can result in injury or death.
Acute Toxic Effects
Follow safe industrial hygiene practices and always wear proper protective equipment when handling this compound.
Continued on Next Page
Propyl Methanesulfonate
Section XII. Ecological Information
Ecotoxicity Not available.
Not available.
Environmental Fate
Section XIII. Disposal Considerations
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material with a
Waste Disposal
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state and local regulations when disposing of the substance.
Section XIV. Transport Information
DOT Classification Not a DOT controlled material (United States).
Not applicable.
PIN Number
Proper Shipping Name Not applicable.
Not applicable.
Packing Group (PG)
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This product is NOT on the EPA Toxic Substances Control Act (TSCA) inventory. The following notices are required by 40
CFR 720.36 (C) for those products not on the inventory list:
(EPA)
(i) These products are supplied solely for use in research and development by or under the supervision of a technically
qualified individual as defined in 40 CFR 720.0 et sec.
(ii) The health risks of these products have not been fully determined. Any information that is or becomes available will be
supplied on an MSDS sheet.
WHMIS Classification Not available.
(Canada)
EINECS Number (EEC) 217-619-9
EEC Risk Statements R20/21/22- Harmful by inhalation, in contact with skin and if swallowed.
R46- May cause heritable genetic damage.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 甲烷磺酸 2-甲基丙基酯 isobutyl methanesulfonate 16156-53-9 C5H12O3S 152.214
反应信息
-
作为反应物:参考文献:名称:Peroxides. I. n-Alkyl Hydroperoxides摘要:DOI:10.1021/ja01640a036
-
作为产物:参考文献:名称:N-烷基化吡唑并[3,4-d]嘧啶类似物的合成及乙酰胆碱酯酶和碳酸酐酶抑制特性的评价摘要:稠合嘧啶,尤其是吡唑并[3,4- d ]嘧啶,是药理学研究中最优选的组成部分,因为它们表现出广泛的生物活性。本研究通过N1氮原子的烷基化合成了吡唑并[3,4- d ]嘧啶的新衍生物。我们合成了3-碘-1 ħ -吡唑并[3,4- d ]嘧啶-4-胺2由市售aminopyrazolopyrimidine 1使用Ñ碘代丁二酰亚胺作为碘化剂。化合物2的合成开始于3-iodo-1 H - pyrazolo[3,4- d的亲核取代]嘧啶-4-胺与R-X(X:-OMs,-Br,-Cl),得到 N-烷基化吡唑并[3,4- d ]嘧啶。我们使用弱无机碱进行这种合成,温和的温度也用于两步程序以生成N-烷基化吡唑并[3,4- d ]嘧啶衍生物。此外,还测试了所有化合物抑制乙酰胆碱酯酶 (AChE) 和人碳酸酐酶 (hCA) 异构体 I 和 II 的能力,AChE 的K i值范围为 15.41 ± 1.39–63DOI:10.1002/ardp.202000330
文献信息
-
Synthesis of Carbonates and Related Compounds from Carbon Dioxide via Methanesulfonyl Carbonates作者:Mark O. Bratt、Paul C. TaylorDOI:10.1021/jo026753g日期:2003.7.1Carbonate anions resulting from reaction of primary or secondary alcohols with carbon dioxide, when added to methanesulfonic anhydride in cooled acetonitrile solution, yield methanesulfonyl carbonates, a new class of synthetic intermediate. Base-mediated reaction of the methanesulfonyl carbonates with alcohols, thiols, and amines yields carbonates, thiocarbonates, and carbamates, respectively. Overall
-
Upper‐Rim Monofunctionalisation in the Synthesis of Triazole‐ and Disulfide‐Linked Multicalix[4]‐ and ‐[6]arenes作者:William H. Gardiner、Matthew Camilleri、Luis A. Martinez‐Lozano、Sean P. Bew、G. Richard StephensonDOI:10.1002/chem.201804755日期:2018.12.17valued in supramolecular chemistry. This work reports an easy and versatile synthetic route to covalently linked double and triple calix[4]arene and calix[6]arenes by a novel DMF‐controlled selective alkylation of a convenient and readily available upper‐rim dimethylaminomethyl‐substituted tetrahydroxy and hexahydroxy calix[4]arene and ‐[6]arenes. Synthetic routes to upper‐rim functionalised redox active
-
[EN] PHENOXYTRIAZOLES<br/>[FR] PHÉNOXYTRIAZOLES申请人:HOFFMANN LA ROCHE公开号:WO2018087018A1公开(公告)日:2018-05-17The present invention relates to a compound of formula I, HetAr is a five or six membered heteroaryl group, selected from wherein R1 is hydrogen, halogen, lower alkyl, lower alkoxy or lower alkyl substituted by halogen, and may be the same or different, if two R1 occur; R2 is lower alkyl or a mono- or polydeutered derivative thereof, lower alkyl substituted by halogen or lower alkoxy, lower alkenyl unsubstituted or substituted by halogen, cycloalkyl or CH2-cycloalkyl unsubstituted or substituted by halogen, heterocycloalkyl or CH2-heterocycloalkyl unsubstituted or substituted by lower alkyl; R3 is halogen, lower alkyl, lower alkyl substituted by halogen, lower alkoxy substituted by halogen or S(0)2-lower alkyl; n is 1, 2 or 3; if n is >1, then R3 may be the same or different; or to a pharmaceutically active acid addition salt thereof, to a racemic mixture or to its corresponding enantiomer and/or an optical isomer and/or stereoisomer thereof. The compounds may be used for the treatment of Alzheimer's disease, cerebral amyloid angiopathy, hereditary cerebral hemorrhage with amyloidosis, Dutch-type (HCHWA-D), multi-infarct dementia, dementia pugilistica or Down syndrome.本发明涉及一种具有式I的化合物,其中HetAr是选择自的五元或六元杂环芳基,其中R1是氢、卤素、较低烷基、较低烷氧基或被卤素取代的较低烷基,且可以相同或不同,如果存在两个R1;R2是较低烷基或其单重或多重氘代衍生物,被卤素取代的较低烷基或较低烷氧基,未取代或被卤素取代的较低烯基,未取代或被卤素取代的环烷基或CH2-环烷基,未取代或被卤素取代的杂环烷基或 -杂环烷基,被较低烷基取代的卤素、较低烷基、被卤素取代的较低烷基、被卤素取代的较低烷氧基或S(0)2-较低烷基;n为1、2或3;如果n>1,则R3可以相同或不同;或其药学活性酸盐,或其外消旋混合物,或其对映体和/或光学异构体和/或立体异构体。这些化合物可用于治疗阿尔茨海默病、脑淀粉样血管病、遗传性脑出血伴淀粉样变性、荷兰型(HCHWA-D)、多梗死性痴呆、拳击性痴呆或唐氏综合征。
-
1-Alkyl-3-methylimidazolium alkanesulfonate ionic liquids, [CnH2n+1mim][CkH2k+1SO3]: synthesis and physicochemical properties作者:Marijana Blesic、Małgorzata Swadźba-Kwaśny、Tayeb Belhocine、H. Q. Nimal Gunaratne、José N. Canongia Lopes、Margarida F. Costa Gomes、Agílio A. H. Pádua、Kenneth R. Seddon、Luís Paulo N. RebeloDOI:10.1039/b910177m日期:——A set of 1-alkyl-3-methylimidazolium alkanesulfonate ionic liquids, [Cnmim][CkSO3], formed by the variation of the alkyl chain lengths both in the cation and the anion (n = 1–6, 8, or 10; k = 1–4, or 6), was synthesised, with sixteen of them being novel. The ionic liquids were characterised by 1H and 13C NMR spectroscopy, and mass spectrometry. Their viscosities and densities as a function of temperature, as well as melting points and decomposition temperatures, were determined. The molecular volumes, both experimental and calculated, were found to depend linearly on the sum (n + k).
-
Method of treating helminthiasis by parenteral or topical administration申请人:E. R. Squibb & Sons, Inc.公开号:US04076825A1公开(公告)日:1978-02-28A method is provided for treating or inhibiting helminthiasis by parenterally or topically administering sulfoxide derivatives of benzimidazoles having the structure ##STR1## wherein R.sup.1 is lower alkyl or phenyl-lower alkyl, and R.sup.2 is lower alkyl. Pharmaceutical compositions for use in the above method are also provided.
表征谱图
-
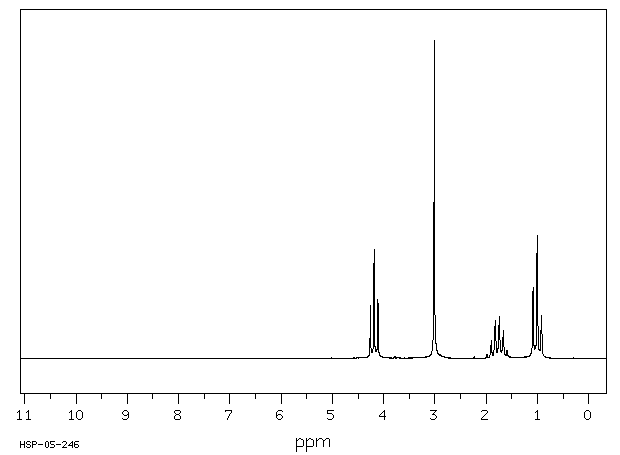
氢谱1HNMR
-
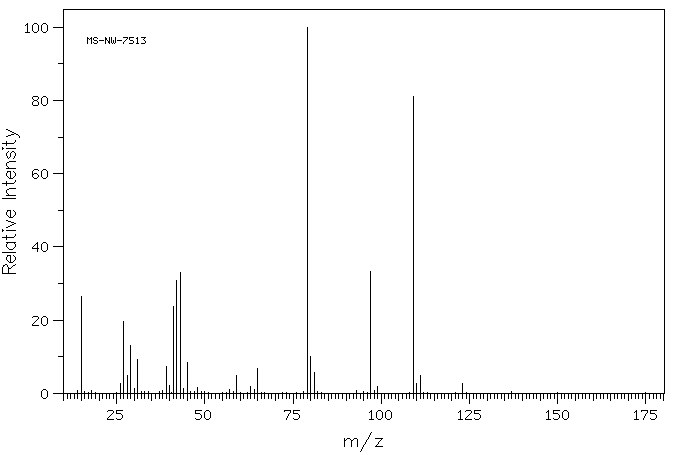
质谱MS
-
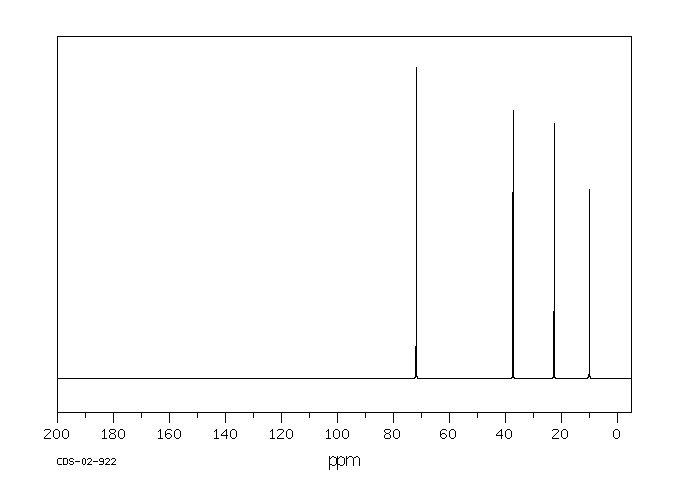
碳谱13CNMR
-
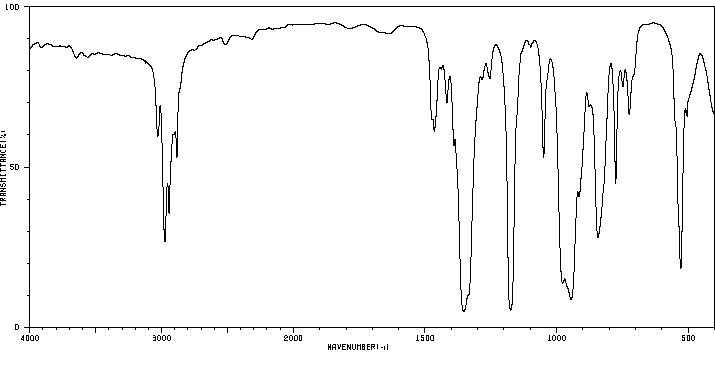
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高烯丙基(甲磺酰基)胺
高炔丙基(甲磺酰基)胺
顺式-9-十八碳烯基甲烷磺酸酯
顺式-4-乙基环己基甲烷磺酸酯
顺-1,2-双(甲磺酰基氧基甲基)环己烷
阿坎酸杂质
阿坎酸
锌甲烷磺酸盐
铵磺酸甜菜碱-3
铵磺酸甜菜碱-2
铵磺酸甜菜碱-1
铬雾抑制剂
铁三(三氟甲基磺酰基)亚胺
钾3-(三羟基硅烷基)-1-丙烷磺酸酯
钾1,1,2,2,3,3,4,4-八氟丁烷-1-磺酸盐
钡二乙烷磺酸酯
钠3-氨基丙烷磺酸酯
钠3-氨基-3-氧代-丙烷-1-磺酸酯
钠3-(三羟基硅烷基)-1-丙烷磺酸酯
钠2-[2-[2-[2-[2-[2-[2-[2-[2-[2-(2-十八碳-9-烯氧基乙氧基)乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]-2-氧代-乙烷磺酸酯
钠1-羟基-1-庚烷磺酸酯
钠(1E)-1-十二碳烯-1-磺酸酯
酪朊酸钠
辛烷-1-磺酸甲酯
辛烷-1-磺酸乙酯
辛基-1-磺酸戊酯
辛-2-烯-1-磺酸
辅酶 M
西尼必利杂质7
萘-1,8-二甲醇
英丙舒凡对甲苯磺酸盐
英丙舒凡
苯基硒基三氟甲烷磺酸酯
芥酸酰胺丙基羟基磺基甜菜碱
艾日布林中间体
脒基牛磺酸
胺磺酸甜菜碱-4
联硫亚盐氯乙醛钠水合物
羧基-五聚乙二醇-磺酸
羟甲基磺酸钠
羟基甲烷磺酸铵盐
羟基甲烷磺酸钾
羟基甲氧基甲醇甲磺酸酯
羟乙磺酸钾
羟乙基磺酸铵盐
羟乙基磺酸钠
羟丙基硫代硫酸钠
美司那
磺酸钠
磺酸己烷