苯六甲酸六甲酯 | 6237-59-8
中文名称
苯六甲酸六甲酯
中文别名
苯六甲酸六甲基酯
英文名称
hexamethyl benzenehexacarboxylate
英文别名
hexamethyl mellitate;hexa(methoxycarbonyl)benzene;benzene-1,2,3,4,5,6-hexacarboxylic acid hexamethyl ester;hexamethyl benzene-1,2,3,4,5,6-hexacarboxylate
CAS
6237-59-8
化学式
C18H18O12
mdl
MFCD00059200
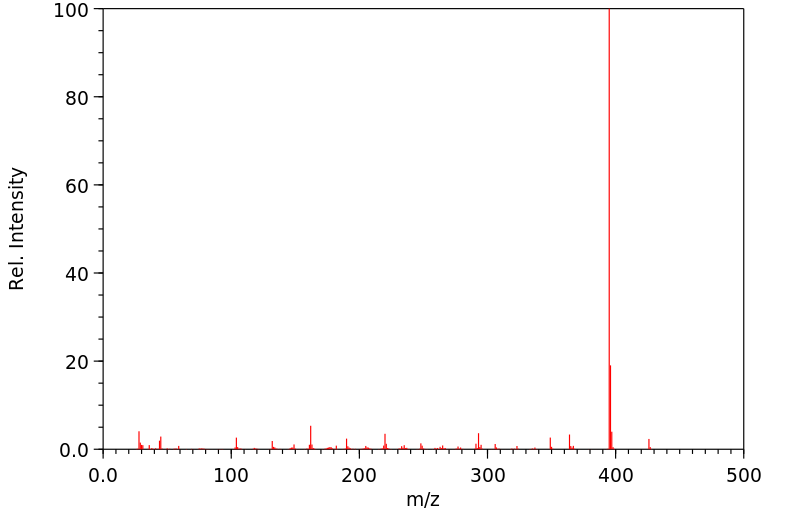
分子量
426.334
InChiKey
BQLICNRRYLYEDI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:190 °C
-
沸点:501.82°C (rough estimate)
-
密度:1.4578 (rough estimate)
-
保留指数:2535;2519.2
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:30
-
可旋转键数:12
-
环数:1.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:158
-
氢给体数:0
-
氢受体数:12
安全信息
-
海关编码:2917399090
-
储存条件:室温
SDS
苯六甲酸六甲酯 修改号码:6
模块 1. 化学品
产品名称: Hexamethyl Benzenehexacarboxylate
修改号码: 6
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 苯六甲酸六甲酯
百分比: >98.0%(GC)
CAS编码: 6237-59-8
俗名: Benzenehexacarboxylic Acid Hexamethyl Ester , Hexamethyl Mellitate ,
Mellitic Acid Hexamethyl Ester
苯六甲酸六甲酯 修改号码:6
模块 3. 成分/组成信息
分子式: C18H18O12
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 固体
外观: 晶体-粉末
颜色: 白色-浅黄色
苯六甲酸六甲酯 修改号码:6
模块 9. 理化特性
气味: 无资料
pH: 无数据资料
熔点:
190°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
苯六甲酸六甲酯 修改号码:6
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Hexamethyl Benzenehexacarboxylate
修改号码: 6
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 苯六甲酸六甲酯
百分比: >98.0%(GC)
CAS编码: 6237-59-8
俗名: Benzenehexacarboxylic Acid Hexamethyl Ester , Hexamethyl Mellitate ,
Mellitic Acid Hexamethyl Ester
苯六甲酸六甲酯 修改号码:6
模块 3. 成分/组成信息
分子式: C18H18O12
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 固体
外观: 晶体-粉末
颜色: 白色-浅黄色
苯六甲酸六甲酯 修改号码:6
模块 9. 理化特性
气味: 无资料
pH: 无数据资料
熔点:
190°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
苯六甲酸六甲酯 修改号码:6
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 苯六甲酸 benzenehexacarboxylic acid 517-60-2 C12H6O12 342.172
反应信息
-
作为反应物:参考文献:名称:苯丙三酰亚胺的简便合成方法摘要:通过用3当量的伯胺处理由偏苯四酸处理得到的固体铵盐,通过脱水和酰亚胺闭环产生三取代的偏苯三酸三酰亚胺。这种令人惊讶的简单合成方法适合于引入烷基,芳基和氨基酸酯取代基,从而为广泛使用C 3对称有机电子受体家族打开了大门。DOI:10.1021/jo800185v
-
作为产物:参考文献:名称:Lindner, Ekkehard; Kuehbauch, Hartwig; Mayer, Hermann A., Chemische Berichte, 1994, vol. 127, # 8, p. 1343 - 1348摘要:DOI:
文献信息
-
Rhodium-Catalyzed Linear Cross-Trimerization of Two Different Alkynes with an Alkene and Two Different Alkenes with an Alkyne作者:Masayuki Kobayashi、Ken TanakaDOI:10.1002/chem.201200903日期:2012.7.23paths with rhodium: A cationic RhI/H8‐BINAP complex has been found to catalyze the linear cross‐trimerization of terminal alkynes, acetylenedicarboxylates, and acrylamides to give substituted trienes. The asymmetric linear cross‐trimerization, giving substituted chiral dienes, has also been achieved by using monosubstituted alkenes and (R)‐BINAP instead of terminal alkynes and H8‐BINAP (see scheme;
-
Exploration of [2 + 2 + 2] cyclotrimerisation methodology to prepare tetrahydroisoquinoline-based compounds with potential aldo–keto reductase 1C3 target affinity作者:Ana R. N. Santos、Helen M. Sheldrake、Ali I. M. Ibrahim、Chhanda Charan Danta、Davide Bonanni、Martina Daga、Simonetta Oliaro-Bosso、Donatella Boschi、Marco L. Lolli、Klaus PorsDOI:10.1039/c9md00201d日期:——
Tetrahydroisoquinoline (THIQ) is a key structural component in many biologically active molecules including natural products and synthetic pharmaceuticals.
-
A Highly Practical <i>Instant</i> Catalyst for Cyclotrimerization of Alkynes to Substituted Benzenes作者:Naoko Saino、Fumihiro Amemiya、Emi Tanabe、Kouki Kase、Sentaro OkamotoDOI:10.1021/ol0602295日期:2006.3.1see text] A 2-(2,6-diisopropylphenyl)iminomethylpyridine (1a)/CoCl(2).6H(2)O/Zn reagent has been developed as an effective instant catalyst for the intra- and intermolecular cyclotrimerization of alkynes to substituted benzenes, making the method extremely practical since the reagent, 1a/CoCl(2).6H(2)O/Zn, is inexpensive and easy to handle and the reaction is less sensitive to moisture and is reasonably
-
Air-Stable {(C<sub>5</sub>H<sub>5</sub>)Co} Catalysts for [2+2+2] Cycloadditions作者:Anaïs Geny、Nicolas Agenet、Laura Iannazzo、Max Malacria、Corinne Aubert、Vincent GandonDOI:10.1002/anie.200806001日期:2009.2.23Cobalt cyclopentadienyl complexes incorporating a fumarate and a CO ligand (see picture) efficiently catalyze inter‐ and intramolecular [2+2+2] cycloadditions of alkynes, nitriles, and/or alkenes to give benzenes, pyridines, or 1,3‐cyclohexadienes. Unlike catalysts such as [CpCo(CO)2] or [CpCo(C2H4)2] (Cp=C5H5), they are air‐stable, easy to handle, compatible with microwave conditions, and do not necessarily
-
CpCo(CO)2-catalysed cyclotrimerisation of alkynes in supercritical carbon dioxide作者:Francisco Montilla、Teresa Avilés、Teresa Casimiro、Ana Aguiar Ricardo、Manuel Nunes da PonteDOI:10.1016/s0022-328x(01)00856-7日期:2001.8complex CpCo(CO)2 as catalyst. The reaction of phenylacetylene produced a mixture of the isomeric cyclotrimers 1,3,5- (2a) and 1,2,4-triphenylbenzene (2a′), in a 1:5 ratio, and traces of cobaltcyclopentadienone complexes CpCo(η4-C4H2[Ph]2CO) (6a, mixture of isomers). The possible product formed by the incorporation of CO2 to alkynes, i.e. diphenylpyrone (7a) was not observed. The reaction of the cobaltacyclopentadiene单取代的HCCR(R = Ph,a ; CH 2 OH,b ; CH 2 CH 2 CH 2 CH 3,c)和二取代的RCCR(R = CH 2 CH 3,d ;使用容易获得的络合物CpCo(CO)2作为催化剂,在超临界二氧化碳(scCO 2)中研究了CO 2 CH 3,e ; Ph,f)乙炔。苯乙炔的反应产生了异构体环三聚体1,3,5-(2a)和1,2,4-三苯苯(图2a' )中,在1:5的比例,并且cobaltcyclopentadienone的痕迹络合物CpCo(η 4 -C 4 H ^ 2 [PH] 2 CO)(图6a,异构体混合物)。未观察到通过将CO 2掺入炔烃而形成的可能产物,即二苯基吡喃酮(7a)。所述cobaltacyclopentadiene的复杂CpCo(1.4-σ-C的反应4 [PH] 4)(PPH)3(8F),在SCCO 2,进行。没有将CO 2插入CoCσ
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
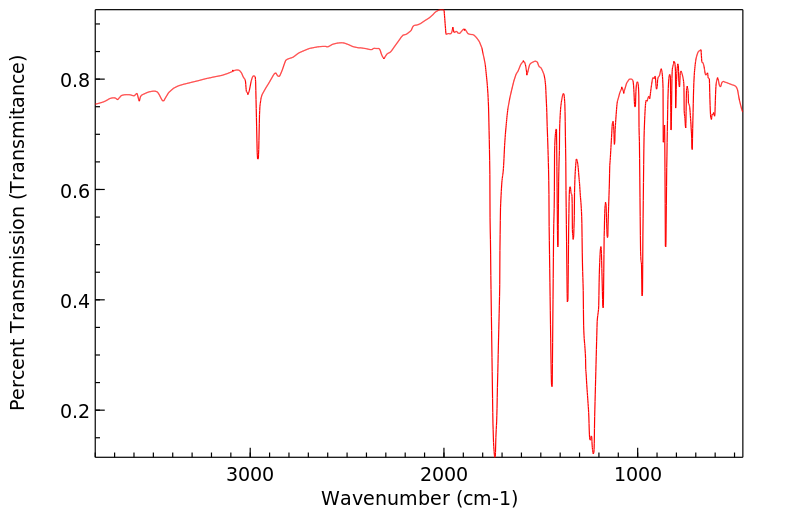
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸