2H-1,2,4-三氮唑-3-甲酰胺 | 3641-08-5
中文名称
2H-1,2,4-三氮唑-3-甲酰胺
中文别名
1,2,4-三氮唑-3-甲酰胺;1,2,4-三氮唑甲酰胺;1H-1,2,4-三唑-3-甲酰胺;1H-1,2,4-三氮唑-3-甲酰胺;3-甲酰胺-1,2,4-三氮唑
英文名称
1,2,4-triazole-3-carboxamide
英文别名
1H-1,2,4-triazole-3-carboxamide;1,2,4-triazole-3-carboxylic acid amide;Triazole carboxamide;1H-1,2,4-triazole-5-carboxamide
CAS
3641-08-5
化学式
C3H4N4O
mdl
MFCD03990481
分子量
112.091
InChiKey
ZEWJFUNFEABPGL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:315 °C
-
沸点:393.4±25.0 °C(Predicted)
-
密度:1.537±0.06 g/cm3(Predicted)
-
溶解度:碱性溶液(微溶),DMSO(微溶)
-
pKa:8.50±0.20 (Predicted,Most Acidic Temp: 25 °C)
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):-0.8
-
重原子数:8
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:84.7
-
氢给体数:2
-
氢受体数:3
安全信息
-
危险品标志:Xi,C
-
WGK Germany:1
-
海关编码:2933990090
-
危险类别:8
-
安全说明:S26,S27,S28,S36/37/39,S45
-
危险类别码:R34
-
包装等级:III
-
危险品运输编号:UN 3263 8/PG 2
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:请将药品存放在避光、通风干燥的地方,并密封保存。
SDS
1,2,4-三氮唑-3-甲酰胺 修改号码:5
模块 1. 化学品
产品名称: 1,2,4-Triazole-3-carboxamide
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 1,2,4-三氮唑-3-甲酰胺
百分比: >97.0%(LC)(N)
CAS编码: 3641-08-5
分子式: C3H4N4O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
1,2,4-三氮唑-3-甲酰胺 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-浅黄色
气味: 无资料
pH: 无数据资料
熔点:
315°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
1,2,4-三氮唑-3-甲酰胺 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
1,2,4-三氮唑-3-甲酰胺 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 1,2,4-Triazole-3-carboxamide
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 1,2,4-三氮唑-3-甲酰胺
百分比: >97.0%(LC)(N)
CAS编码: 3641-08-5
分子式: C3H4N4O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
1,2,4-三氮唑-3-甲酰胺 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-浅黄色
气味: 无资料
pH: 无数据资料
熔点:
315°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
1,2,4-三氮唑-3-甲酰胺 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
1,2,4-三氮唑-3-甲酰胺 修改号码:5
模块16 - 其他信息
N/A
制备方法与用途
制备方法
医药中间体。
用途简介反应信息
-
作为反应物:描述:参考文献:名称:等位利巴韦林类似物:合成和抗病毒活性摘要:通过两种不同的方式合成了新型的等位性利巴韦林类似物。其中一些对丙型肝炎病毒(HCV),单纯疱疹(HCV-1)和A型流感病毒显示了与病毒唑本身相当的抗病毒作用。获得的数据证实了与生物等位基因有关的利巴韦林可能的抗病毒活性机制的理论建议。DOI:10.1016/j.bmcl.2017.11.029
-
作为产物:描述:利巴韦林 在 human blood purine nucleoside phosphorylase 作用下, 以 phosphate buffer 为溶剂, 反应 0.17h, 生成 2H-1,2,4-三氮唑-3-甲酰胺参考文献:名称:Dual-Action Mechanism of Viramidine Functioning as a Prodrug and as a Catabolic Inhibitor for Ribavirin摘要:摘要 最近出现了一种正在研究的核苷类似物药物--维拉米定,它可能是治疗丙型肝炎病毒感染的利巴韦林的一种更安全的替代药物。我们曾报道过,维拉米定主要作为利巴韦林的原药在肝脏中富集。这项体外研究进一步探讨了紫杉烷对核苷磷酸化酶的活性,核苷磷酸化酶是一种宿主酶,负责体内利巴韦林的磷酸化。我们的实验表明,紫杉烷对利巴韦林磷酸化的抑制率为 K i 为 2.5 μM。这一结果表明,紫杉烷可能通过双重作用机制发挥作用,既是利巴韦林的原药,又是利巴韦林核苷磷酸酶分解的抑制剂。DOI:10.1128/aac.48.10.4006-4008.2004
文献信息
-
Synthesis of 5,5-difluoro-5-phosphono-pent-2-en-1-yl nucleosides as potential antiviral agents作者:F. Chevrier、Z. Chamas、T. Lequeux、E. Pfund、G. Andrei、R. Snoeck、V. Roy、L. A. AgrofoglioDOI:10.1039/c7ra05153k日期:——A series of hitherto unknown acyclic 5,5-difluoro-5-phosphono-pent-2-en-1-yl-pyrimidines (9a, b, 13a, b), -purines (16a, b) and -(1,2,4)-triazolo-3-carboxamide (19) were successfully synthesized from (E)-1-bromo-5-diethoxyphosphoryl-5,5-difluoro-pent-2-ene in a stereoselective manner. All the synthetized compounds were assayed for antiviral activity against various viruses, but were found to be neither
-
Synthesis and broad spectrum antiviral evaluation of bis(POM) prodrugs of novel acyclic nucleosides作者:Manabu Hamada、Vincent Roy、Tamara R. McBrayer、Tony Whitaker、Cesar Urbina-Blanco、Steven P. Nolan、Jan Balzarini、Robert Snoeck、Graciela Andrei、Raymond F. Schinazi、Luigi A. AgrofoglioDOI:10.1016/j.ejmech.2013.06.053日期:2013.9synthetic step. All novel compounds were evaluated for their antiviral activities against a large number of DNA and RNA viruses including herpes simplex virus type 1 and 2, varicella zoster virus, feline herpes virus, human cytomegalovirus, hepatitis C virus (HCV), HIV-1 and HIV-2. Among these molecules, only compound 31 showed activity against human cytomegalovirus in HEL cell cultures with at EC50一系列迄今未知的 ANP 类似物,带有 ( E )-but-2-enyl 脂肪族侧链和修饰的杂环碱基,例如胞嘧啶和 5-氟胞嘧啶、2-吡嗪甲酰胺、1,2,4-三唑-3-甲酰胺或4-取代-1,2,3-三唑通过烯烃无环交叉复分解作为关键合成步骤直接制备。 评估了所有新化合物对大量 DNA 和 RNA 病毒的抗病毒活性,包括单纯疱疹病毒 1 型和 2 型、水痘带状疱疹病毒、猫疱疹病毒、人类巨细胞病毒、丙型肝炎病毒 (HCV)、HIV-1 和 HIV -2。在这些分子中,只有化合物31在 HEL 细胞培养物中显示出对人巨细胞病毒的活性,EC 50为~10 μM。化合物8a、13、14和24在100 μM时表现出显着的抗 HCV 活性而没有显着的细胞毒性。
-
Purification and properties of purine nucleoside phosphorylase from Brevibacterium acetylicum ATCC 954.作者:Hideyuki SHIRAE、Kenzo YOKOZEKIDOI:10.1271/bbb1961.55.493日期:——Purine nucleoside phosphorylase of Brevibacterium acetylicum ATCC 954, which catalyzes the production of ribavirin (1-β-D-ribofuranosyl-1, 2, 4-triazole-3-carboxamide), a potent antiviral agent, from purine nucleoside and 1, 2, 4-triazole-3-carboxamide in a high yield, was purified 49-fold. This enzyme had a molecular weight of 31, 000 and was a monomer. The isoelectric point of the enzyme was 4.7. The optimal temperature and pH of inosine phosphorolyzing reaction catalyzed by the enzyme was around 8.5 and 70°C, respectively. The Michaelis constants for inosine, guanosine, and ribavirin were 1.43 mM, 2.44 mM and 2.08 mM, respectively, at 40°C. This enzyme appeared to be a SH enzyme because it was inactivated by SH reagents, p-chloromercuribenzoate and N-ethylmaleimide, and HgCl2. In addition, this enzyme was completely inactivated by AgNO3 and was slightly inhibited by CuSO4. It showed nucleoside-phosphorolyzing activity toward inosine, 2'-deoxyinosine, 2', 3'-dideoxyinosine, guanosine, 2'-deoxyguanosine, and xanthosine. However, adenosine and its derivatives could not be phosphorolyzed. This enzyme could not also phosphorolyze various 5'-mononucleotides. According to the amino terminal sequence analysis, the twenty residues from the amino terminal end of this enzyme were identified as follows: MTVNWNETRS-FLECKMQAKPE.Brevibacterium acetylicum ATCC 954 的嘌呤核苷磷酸化酶能够以高产率催化从嘌呤核苷和 1, 2, 4-三氮唑-3-羧酰胺生成强效抗病毒剂利巴韦林(1-β-D-核糖苷-1, 2, 4-三氮唑-3-羧酰胺),其已被纯化49倍。该酶的分子量为31,000,属于单体。该酶的等电点为4.7。该酶催化的肌苷磷酸水解反应的最佳温度和pH分别为约70°C和8.5。肌苷、鸟苷和利巴韦林的米氏常数分别为1.43 mM、2.44 mM和2.08 mM(在40°C下)。该酶似乎是一种SH酶,因为它被SH试剂、对氯汞苯甲酸酯和N-乙基马来酰亚胺以及HgCl2失活。此外,该酶被AgNO3完全失活,并且对CuSO4有轻微抑制作用。它对肌苷、2'-去氧肌苷、2', 3'-二脱氧肌苷、鸟苷、2'-去氧鸟苷和黄苷显示出核苷磷酸化活性。然而,腺苷及其衍生物不能被磷酸化。该酶也无法磷酸化各种5'-单核苷酸。根据氨基端序列分析,这个酶的氨基端20个残基被确认为:MTVNWNETRS-FLECKMQAKPE。
-
Chemoenzymatic method of 1,2,4-triazole nucleoside synthesis: Possibilities and limitations作者:I. D. Konstantinova、M. V. Chudinov、I. V. Fateev、A. V. Matveev、N. I. Zhurilo、V. I. Shvets、A. I. MiroshnikovDOI:10.1134/s1068162013010056日期:2013.1of novel structural analogues of an antiviral preparation of Ribavirin (1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide) were established. A synthesis of various amides of 1H-1,2,4-triazole-3-carboxylic acid and its 5-substituted analogues—potential substrates of purine nucleoside phosphorylase—has been described. Comparative efficiency of preparation methods of these amides, as well as the methods
-
Acetoxy Meldrum’s Acid: A Versatile Acyl Anion Equivalent in the Pd-Catalyzed Asymmetric Allylic Alkylation作者:Barry M. Trost、Maksim Osipov、Philip S. J. Kaib、Mark T. SorumDOI:10.1021/ol2011242日期:2011.6.17Acetoxy Meldrum’s acid can serve as a versatile acyl anion equivalent in the Pd-catalyzed asymmetric allylic alkylation. The reaction of this nucleophile with various meso and racemic electrophiles afforded alkylated products in high yields and enantiopurities. These enantioenriched products are versatile intermediates that can be further functionalized using nitrogen- and oxygen-centered nucleophilesAcetoxy Meldrum 的酸可以在 Pd 催化的不对称烯丙基烷基化中用作通用的酰基阴离子等价物。该亲核试剂与各种内消旋和外消旋亲电试剂的反应以高产率和对映纯度提供烷基化产物。这些对映体富集的产品是多功能中间体,可以使用以氮和氧为中心的亲核试剂进一步功能化,为合成核苷类似物提供多功能支架。这些支架用于完成抗 HIV 药物卡波韦、阿巴卡韦和抗生素阿里斯特霉素的正式合成。
表征谱图
-
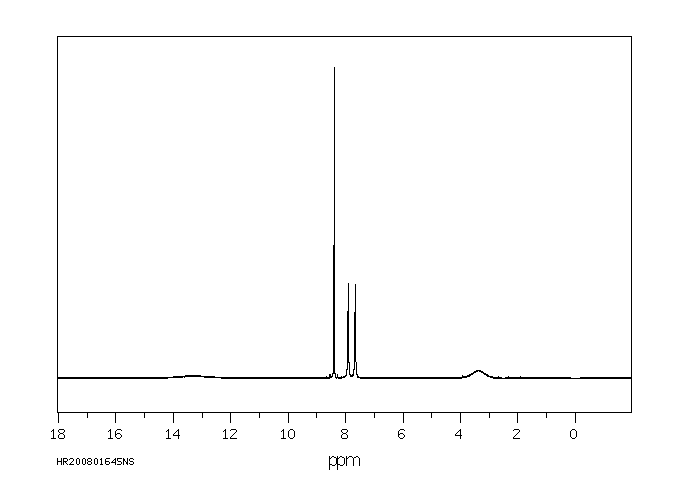
氢谱1HNMR
-
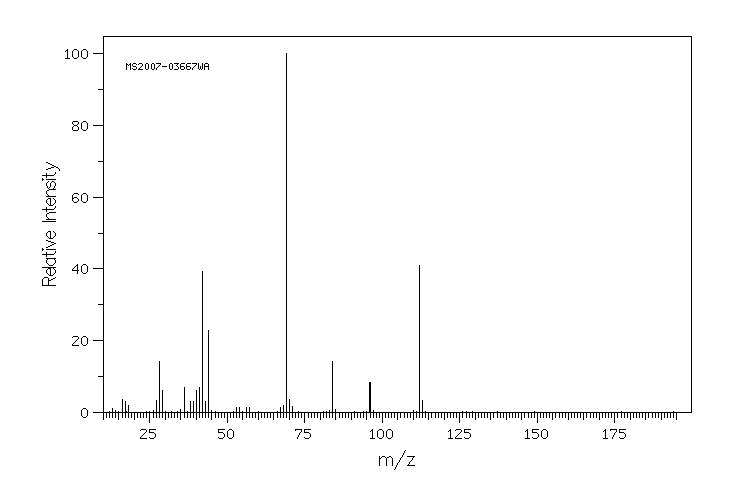
质谱MS
-
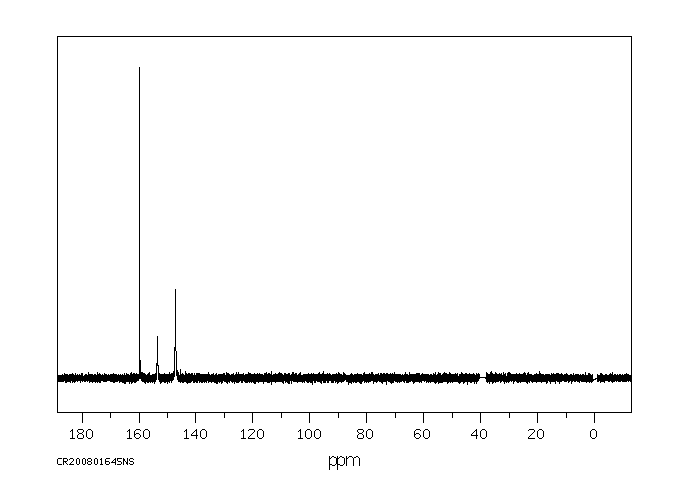
碳谱13CNMR
-
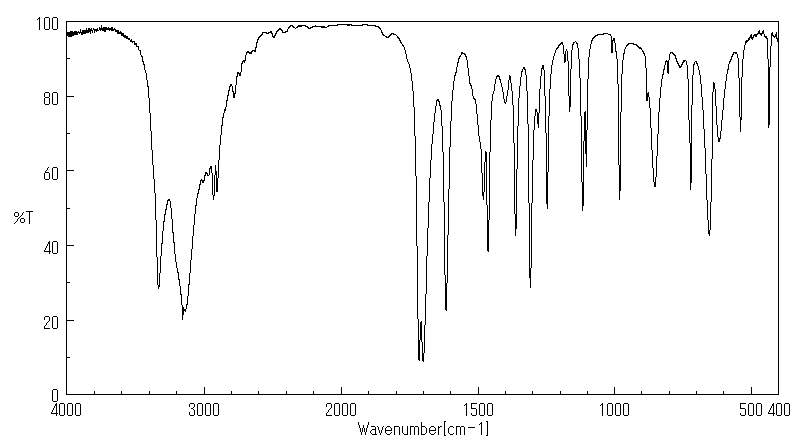
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸