间丁基苯硼酸 | 560132-24-3
中文名称
间丁基苯硼酸
中文别名
3-叔丁基苯硼酸
英文名称
3-tert-butylphenylboronic acid
英文别名
3-t-butylphenylboronic acid;3-tert-butylbenzeneboronic acid;m-(tert-butyl)phenylboronic acid;(3-tert-butylphenyl)boronic acid
CAS
560132-24-3
化学式
C10H15BO2
mdl
MFCD11110647
分子量
178.039
InChiKey
OKBOGYOXEDEGOG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:221 °C(Solv: water (7732-18-5))
-
沸点:304.3±35.0 °C(Predicted)
-
密度:1.02±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.27
-
重原子数:13
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:2
安全信息
-
安全说明:S26,S39
-
危险类别码:R41
-
海关编码:2931900090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:室温条件下。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 3-tert-Butylphenylboronic acid
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 3-tert-Butylphenylboronic acid
CAS number: 560132-24-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C10H15BO2
Molecular weight: 178.0
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 3-tert-Butylphenylboronic acid
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 3-tert-Butylphenylboronic acid
CAS number: 560132-24-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C10H15BO2
Molecular weight: 178.0
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:间丁基苯硼酸 在 palladium diacetate 、 potassium carbonate 、 三苯基膦 作用下, 以 乙二醇二甲醚 为溶剂, 反应 34.0h, 生成 N-formyl-2-amino-3′,5-di-tert-butylbiphenyl参考文献:名称:시클로금속화 이미다조[1,2-f]페난트리딘 및 디이미다조[1,2-a:1',2'-c]퀴나졸린 리간드, 및 이의 등전자성 및 벤즈고리화된 유사체의 금속 착체摘要:This is the translation of the text into Chinese: 本发明涉及包含环金属化咔唑[1,2-f]苯并三氮杂[1,2-a:1',2'-c]喹唑啉配体,或其等电子性或苯环化类似体的化合物,其中包括光电金属配位体。此外,本发明涉及包含这些化合物的有机发光二极管器件。公开号:KR20160030582A
-
作为产物:参考文献:名称:三重融合的卟啉-纳米碳烯共轭物的合成摘要:通过Scholl反应,由基于苯并[ m ]四苯并菲基卟啉的量身定制的前体合成了两个前所未有的卟啉稠合纳米石墨烯分子1和2。化学结构通过高分辨率基质辅助激光解吸/电离飞行时间质谱(HR MALDI-TOF MS),IR和拉曼光谱以及扫描隧道显微镜(STM)的组合进行了验证。1和2的近紫外可见吸收光谱表明,宽广的红移吸收光谱分别扩展到1000和1400 nm,这标志着π共轭体系的显着扩展。DOI:10.1002/anie.201805063
文献信息
-
一种含硼有机电致发光化合物及其在有机电致发光器件上的应用
-
Ru-Catalysed CH Arylation of Indoles and Pyrroles with Boronic Acids: Scope and Mechanistic Studies作者:Carina Sollert、Karthik Devaraj、Andreas Orthaber、Paul J. Gates、Lukasz T. PilarskiDOI:10.1002/chem.201405931日期:2015.3.27The Ru‐catalysed C2H arylation of indoles and pyrroles by using boronic acids under oxidative conditions is reported. This reaction can be applied to tryptophan derivatives and tolerates a wide range of functional groups on both coupling partners, including bromides and iodides, which can be further derivatised selectively. New indole‐based ruthenacyclic complexes are described and investigated as
-
All Non‐Carbon B <sub>3</sub> NO <sub>2</sub> Exotic Heterocycles: Synthesis, Dynamics, and Catalysis作者:Christopher R. Opie、Hidetoshi Noda、Masakatsu Shibasaki、Naoya KumagaiDOI:10.1002/chem.201900715日期:2019.3.27The B3NO2 six‐membered heterocycle (1,3‐dioxa‐5‐aza‐2,4,6‐triborinane=DATB), comprising three different non‐carbon period 2 elements, has been recently demonstrated to be a powerful catalyst for dehydrative condensation of carboxylic acids and amines. The tedious synthesis of DATB, however, has significantly diminished its utility as a catalyst, and thus the inherent chemical properties of the ringB 3 NO 2六元杂环(1,3-二氧杂-5-氮杂-2,4,6-三硼烷= DATB),由三种不同的非碳2期元素组成,最近被证明是羧酸脱水缩合的强大催化剂酸和胺。然而,繁琐的DATB合成大大降低了其作为催化剂的用途,因此,实际上仍未探索环系统的固有化学性质。在此公开了一种通用且简便的合成策略,该策略利用含嘧啶的支架可靠地安装硼原子,从而以模块化的方式由廉价材料制成了一系列Pym-DATB。可溶性Pym‐DATB衍生物的鉴定可用于研究B 3 NO 2的动力学性质环系统,根据介质的不同,显示出不同的开环和开环行为。易于获取的Pym‐DATB证明了其作为脱水酰胺化的有效催化剂的实用性,具有广泛的底物范围和官能团耐受性,为试剂驱动的酰胺化提供了一种通用且实用的催化替代方法。
-
Organic electroluminescent materials and devices申请人:Universal Display Corporation公开号:US09281483B2公开(公告)日:2016-03-08Compounds comprising phosphorescent metal complexes comprising cyclometallated imidazo[1,2-f]phenanthridine and diimidazo[1,2-a:1′,2′-c]quinazoline ligands, or isoelectronic or benzannulated analogs thereof, are described. Organic light emitting diode devices comprising these compounds are also described.
-
Spin-Center Shift-Enabled Direct Enantioselective α-Benzylation of Aldehydes with Alcohols作者:Eric D. Nacsa、David W. C. MacMillanDOI:10.1021/jacs.7b12768日期:2018.3.7two-electron pathways. We report herein an enantioselective α-benzylation of aldehydes using alcohols as alkylating agents based on the mechanistic principle of spin-center shift. This strategy harnesses the dual activation modes of photoredox and organocatalysis, engaging the alcohol by SCS and capturing the resulting benzylic radical with a catalytically generated enamine. Mechanistic studies provide
表征谱图
-
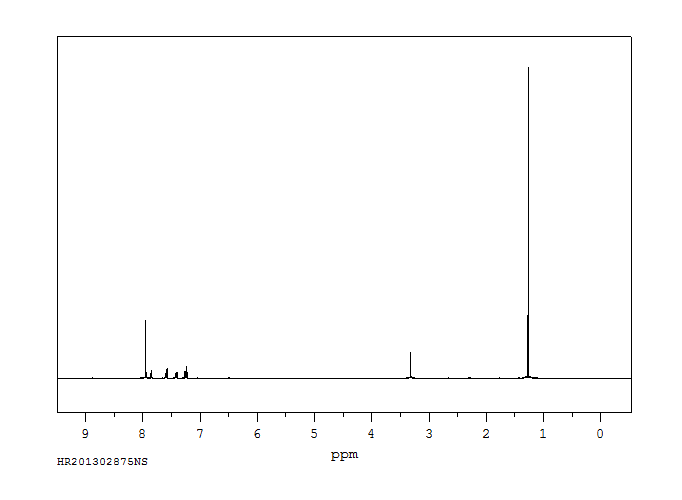
氢谱1HNMR
-
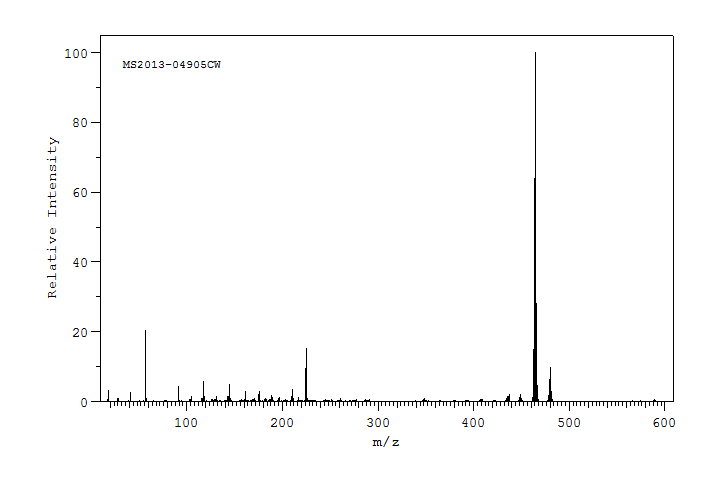
质谱MS
-
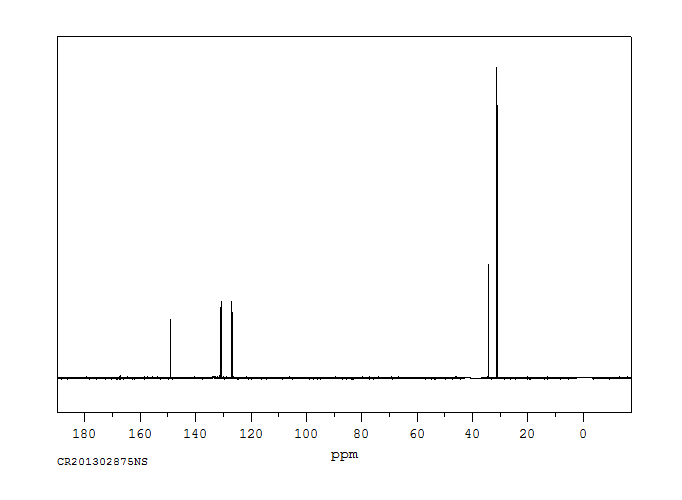
碳谱13CNMR
-
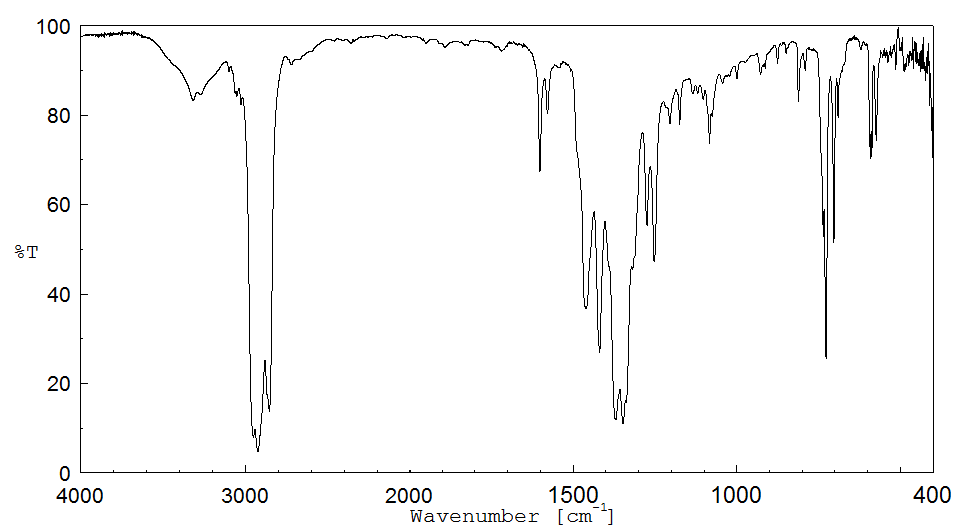
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫