1-异喹啉硼酸 | 3696-36-4
中文名称
1-异喹啉硼酸
中文别名
奥麦丁二硫化物;2,2'-二硫代二(吡啶-1-氧化物);双硫氧吡啶;2-甲基丙二腈
英文名称
2-methylmalononitrile
英文别名
methylmalononitrile;2-methylpropanedinitrile;2-methylmalonitrile
CAS
3696-36-4
化学式
C4H4N2
mdl
MFCD01715963
分子量
80.0892
InChiKey
LXUTYOVUICAOGH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:36-37 °C(Solv: benzene (71-43-2); ligroine (8032-32-4))
-
沸点:90-100 °C(Press: 15 Torr)
-
密度:0.980±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):0.4
-
重原子数:6
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:47.6
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2926909090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 丙二腈 malononitrile 109-77-3 C3H2N2 66.0623
反应信息
-
作为反应物:参考文献:名称:非共轭无环自由基反应中的 1,2-不对称诱导:烷基取代自由基高选择性原子转移反应的新模型摘要:据报道,甲基碘代丙二腈 (1) 通过碘转移加成到几种二烷基取代的烯烃上。由于 1,2-不对称诱导,碘转移反应到无环非共轭自由基中间体通常会导致选择性产物形成。非对映选择性的水平取决于基团上烷基取代基的大小。叔烷基产生高顺式选择性,仲基产生中等顺式选择性,伯基显示完全非选择性反应。为了解释立体化学结果,基于 AM1 计算和 EPR 数据引入了 1 Bu 取代基 5e 的新空间模型DOI:10.1021/ja00072a010
-
作为产物:描述:参考文献:名称:Wallenfels,K. et al., Justus Liebigs Annalen der Chemie, 1976, p. 656 - 665摘要:DOI:
-
作为试剂:描述:2,2-dimethyl-1-(5'-nitro-2'-furyl)-1-propanol 在 sodium tetrahydroborate 、 氯化亚砜 、 1-异喹啉硼酸 作用下, 以 乙醇 、 N,N-二甲基甲酰胺 为溶剂, 反应 5.0h, 生成 2-<2',2'-Dimethyl-1'-(5''-nitro-2''-furyl)propyl>-2-methyl-malononitrile参考文献:名称:SRN1 reactions in nitrofuran derivatives摘要:DOI:10.1021/jo00389a028
文献信息
-
Ruthenium-Catalyzed Regioselective Reactions of Nitriles and 1,3-Dicarbonyl Compounds with Terminal Alkynes¹作者:Shun-Ichi Murahashi、Takeshi Naota、Yoshinori NakanoDOI:10.1055/s-0029-1218373日期:2009.12RuH 2 (PPh 3 ) 4 -catalyzed reaction of nitriles with terminal alkynes proceeds highly efficiently under neutral conditions to give the corresponding Michael adducts. Furthermore, 1,3-dicarbonyl compounds react with terminal alkynes at the α-position to afford the exo-methylene compounds with high regioselectivity under neutral conditions. The regioselectivity depends upon the change of substrates
-
Palladium catalysed direct allylation of pronucleophiles with allylstannanes作者:Yoshinori Yamamoto、Naoya FujiwaraDOI:10.1039/c39950002013日期:——The reaction of pronucleophiles 1 with allyltributylstannanes in the presence of catalytic amounts of Pd2(dba)3·CHCl3(4 mol%) and 1,2-bis(diphenylphosphino)ethane (dppe)(10 mol%) at room temperature gives the corresponding allylation products in good to high yields.
-
Enantioselective Synthesis of Tertiary Allylic Fluorides by Iridium‐Catalyzed Allylic Fluoroalkylation作者:Trevor W. Butcher、John F. HartwigDOI:10.1002/anie.201807474日期:2018.10Few allylic electrophiles containing two different substituents at a single allyl terminus and none in which one of the two substituents is a heteroatom, have been shown previously to react with iridium catalysts to form substitution products. We report that iridium‐catalysts are uniquely suited to form tertiary allylic fluorides enantioselectively by the addition of a diverse range of carbon‐centered
-
Carbopalladation of Nitriles: Synthesis of Benzocyclic Ketones and Cyclopentenones via Pd-Catalyzed Cyclization of ω-(2-Iodoaryl)alkanenitriles and Related Compounds作者:Alexandre A. Pletnev、Richard C. LarockDOI:10.1021/jo0262006日期:2002.12.1An efficient procedure for the synthesis of 2,2-disubstituted benzocyclic ketones by intramolecular carbopalladation of nitriles has been developed. The cyclization of substituted 3-(2-iodoaryl)propanenitriles affords indanones in high yields. The reaction is compatible with a wide variety of functional groups. This methodology has been extended to the synthesis of tetralones and cyclopentenones.
-
腈及其相应胺的制造方法申请人:中国石化扬子石油化工有限公司公开号:CN105001033B公开(公告)日:2018-06-22本发明涉及一种腈的制造方法,与现有技术相比,具有氨源用量显著降低、环境压力小、能耗低、生产成本低、腈产品的纯度和收率高等特点,并且能够获得结构更为复杂的腈。本发明还涉及由该腈制造相应胺的方法。
表征谱图
-
氢谱1HNMR
-
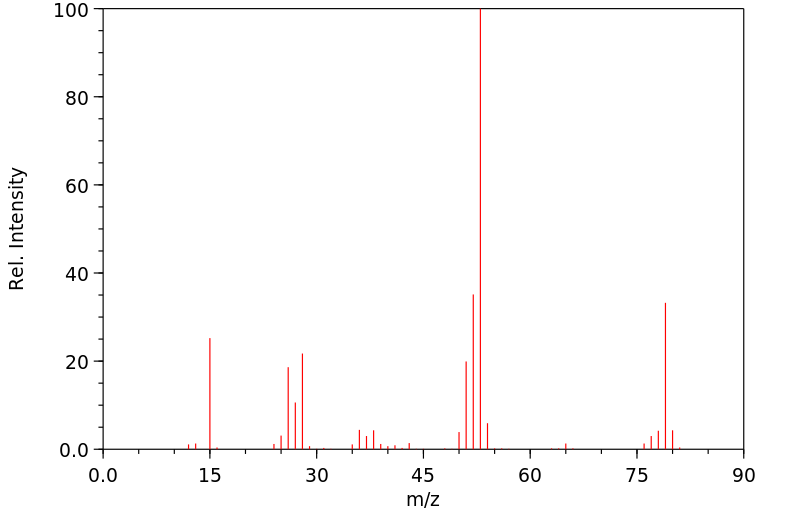
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷