2-甲基己二酸 | 626-70-0
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:64°C
-
沸点:328.8°C (rough estimate)
-
密度:1.3681 (rough estimate)
-
物理描述:Solid
计算性质
-
辛醇/水分配系数(LogP):0.5
-
重原子数:11
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.714
-
拓扑面积:74.6
-
氢给体数:2
-
氢受体数:4
SDS
| Name: | 2-Methyladipic Acid Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 626-70-0 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 626-70-0 | 2-Methyladipic Acid | ca. 100 | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Can produce delayed pulmonary edema.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation. Wash clothing before reuse.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 626-70-0: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 88 - 92 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: Insoluble.
Specific Gravity/Density:
Molecular Formula: C7H12O4
Molecular Weight: 160.17
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Dust generation.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 626-70-0 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2-Methyladipic Acid - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 626-70-0: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 626-70-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 626-70-0 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-甲基己二酸二乙酯 diethyl 2-methylhexanedioate 91214-21-0 C11H20O4 216.277 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-甲基己二酸二甲酯 dimethyl 2-methyladipate 66432-76-6 C9H16O4 188.224
反应信息
-
作为反应物:描述:参考文献:名称:Grundmann; Loew, Hoppe-Seyler's Zeitschrift fur Physiologische Chemie, 1938, vol. 256, p. 141,151摘要:DOI:
-
作为产物:描述:参考文献:名称:Smith, John R. Lindsay; Thomas, C. Barry; Whittaker, Mark, Journal of the Chemical Society. Perkin transactions II, 1993, # 11, p. 2191 - 2194摘要:DOI:
文献信息
-
SYNTHÈSES D'ACIDES AMINÉS CYCLIQUES À PARTIR DE DÉRIVÉS DE L'ACIDE ADIPIQUE作者:Lise Nicole、Louis BerlinguetDOI:10.1139/v62-054日期:1962.2.1
The Dieckmann reaction has been reinvestigated in view of obtaining alkyl-substituted cyclopentanones. Control of the reaction during alkylation now permits either the opening of the cyclopentane ring to give, without the isolation of intermediates, substituted adipic esters in excellent yields or normal substitution on the ring.Methyl- and allyl-substituted cyclopentanones were obtained from the corresponding Dieckmann esters or from the substituted adipic acids. These ketones were transformed by the Strecker method into their corresponding spirohydantoins. In both cases, two diastereo-isomers were isolated and studied.1-Amino-2-methyl-cyclopentanecarboxylic acid and 1-amino-2-allyl-cyclopentanecarboxylic acid were prepared in good yields from the spirohydantoins and characterized.
-
[EN] LPXH TARGETING COMPOUNDS, COMPOSITIONS THEREOF, AND METHODS OF MAKING AND USING THE SAME<br/>[FR] COMPOSÉS DE CIBLAGE LPXH, LEURS COMPOSITIONS ET PROCÉDÉS DE FABRICATION ET D'UTILISATION ASSOCIÉS申请人:UNIV DUKE公开号:WO2021072369A1公开(公告)日:2021-04-15LpxH targeting compounds, compositions thereof, as well as methods for for making and using the same are disclosed herein. The LpxH target compounds typically have a structure pursuant to Formula (I) and/or a salt thereof, wherein Rb is selected from a single bond, C4 to C10 unsubstituted aryl, C4 to C10 substituted aryl, unsubstituted or substituted four to ten member heterocycle ring, C1 to C10 unsubstituted alkyl, and C1 to C10 substituted alkyl; Rc comprises hydrogen, halogen, -OH, -CO2CH3, -COOH, -CN2CF3, -CF3,-C2OH, -CONHOH, -CCOH, C4 to C10 unsubstituted aryl, C4 to C10 substituted aryl, unsubstituted or substituted four to ten member heterocycle ring, C1 to C10 unsubstituted alkyl, or C1 to C10 substituted alkyl; and Rd and Re are independently hydrogen, -OH, -COH, -COH, -COC, -COOH, Rf, or are taken together as an unsubstituted or substituted four to eight member nitrogen containing heterocycle ring.LpxH靶向化合物,其组合物,以及制备和使用这些化合物的方法在此披露。LpxH靶向化合物通常具有符合式(I)的结构和/或其盐,其中Rb从单键,C4到C10未取代芳基,C4到C10取代芳基,未取代或取代的四到十元杂环环,C1到C10未取代烷基,和C1到C10取代烷基中选择;Rc包括氢,卤素,-OH,-CO2CH3,-COOH,-CN2 ,-CF3,-C2OH,-CONHOH,-CCOH,C4到C10未取代芳基,C4到C10取代芳基,未取代或取代的四到十元杂环环,C1到C10未取代烷基,或C1到C10取代烷基;而Rd和Re独立地是氢,-OH,-COH,-COH,-COC,-COOH,Rf,或作为未取代或取代的四到八元含氮杂环环一起取代。
-
Ni-Catalyzed Site-Selective Dicarboxylation of 1,3-Dienes with CO<sub>2</sub>作者:Andreu Tortajada、Ryo Ninokata、Ruben MartinDOI:10.1021/jacs.7b13220日期:2018.2.14A site-selective catalytic incorporation of multiple CO2 molecules into 1,3-dienes en route to adipic acids is described. This protocol is characterized by its mild conditions, excellent chemo- and regioselectivity and ease of execution under CO2 (1 atm), including the use of bulk butadiene and/or isoprene feedstocks.
-
[EN] CYTOTOXIC BIS-BENZODIAZEPINE DERIVATIVES AND CONJUGATES THEREOF WITH CELL-BINDING AGENTS FOR INHIBITING ABNORMAL CELL GROWTH OR FOR TREATING PROLIFERATIVE DISEASES<br/>[FR] DÉRIVÉS DE BIS-BENZODIAZÉPINE CYTOTOXIQUES ET LEURS CONJUGUÉS AVEC DES AGENTS DE LIAISON À UNE CELLULE POUR INHIBER LA CROISSANCE CELLULAIRE ANORMALE OU POUR TRAITER DES MALADIES PROLIFÉRATIVES申请人:IMMUNOGEN INC公开号:WO2020205564A1公开(公告)日:2020-10-08The invention relates to benzodiazepine derivatives with antiproliferative activity and more specifically to benzodiazepine compounds of formulae (I), (II), (TI) and (T2). The invention also provides conjugates of the benzodiazepine compounds linked to a cell-binding agent. The invention further provides compositions and methods for inhibiting abnormal cell growth or treating a proliferative disorder in a mammal using the compounds or conjugates of the invention.这项发明涉及具有抗增殖活性的苯二氮䓬啶衍生物,更具体地涉及具有式(I)、(II)、(TI)和(T2)的苯二氮䓬啶化合物。该发明还提供了与细胞结合剂连接的苯二氮䓬啶化合物的结合物。该发明还提供了使用该发明的化合物或结合物抑制异常细胞生长或治疗哺乳动物增殖性疾病的组合物和方法。
-
Compound having silsesquioxane skeleton and its polymer申请人:Inagaki Jyun-ichi公开号:US20050009982A1公开(公告)日:2005-01-13The present invention relates to a compound represented by Formula (1) and a polymer obtained using the compound: wherein R 1 is phenyl which may have substituents, Q 1 is hydrogen, halogen, alkyl having 1 to 10 carbon atoms, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclohexenyl or phenyl in which optional hydrogen may be replaced by halogen or alkyl having 1 to 5 carbon atoms, and Q 2 is a group represented by Formula (2) wherein the code < represents a bonding point with silicon, l, m, n and p are independently 0, 1, 2 or 3, A 1 to A 4 are independently a single bond, 1,4-cyclohexylene, 1,4-cyclohexenylene, a condensed ring group having 6 to 10 carbon atoms which is a divalent group, or 1,4-phenylene, Z 0 to Z 3 are independently a single bond, —CH═CR—, —C≡C—, —COO—, —OCO—, or alkylene having 1 to 20 carbon atoms, and Z 4 is a single bond, —CH═CH—, —C≡C—, —COO—, —OCO—, or alkylene having 1 to 20 carbon atoms. And Y 1 in Formula (1) is the group defined in Claim 1.本发明涉及一种由式(1)表示的化合物和使用该化合物获得的聚合物:其中R1是苯基,可能具有取代基;Q1是氢、卤素、具有1至10个碳原子的烷基、环丙基、环丁基、环戊基、环己基、环己烯基或苯基,其中可选的氢原子可被卤素或具有1至5个碳原子的烷基取代;Q2是由式(2)表示的基团,其中代码<表示与硅的连接点,l、m、n和p独立地为0、1、2或3,A1至A4独立地为单键、1,4-环己亚基、1,4-环己烯亚基、具有6至10个碳原子的缩合环基团,为二价基团,或1,4-苯亚基,Z0至Z3独立地为单键、—CH═CR—、—C≡C—、—COO—、—OCO—或具有1至20个碳原子的烷基,Z4为单键、—CH═CH—、—C≡C—、—COO—、—OCO—或具有1至20个碳原子的烷基。式(1)中的Y1是权利要求1中定义的基团。
表征谱图
-
氢谱1HNMR
-
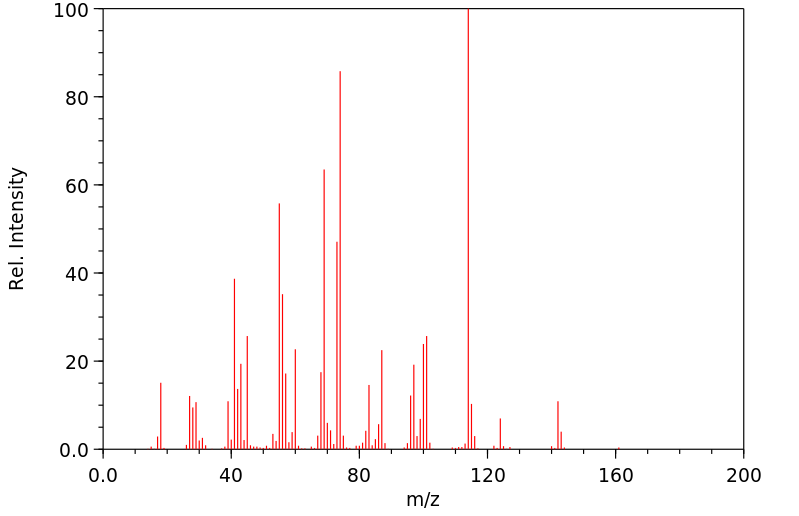
质谱MS
-
碳谱13CNMR
-
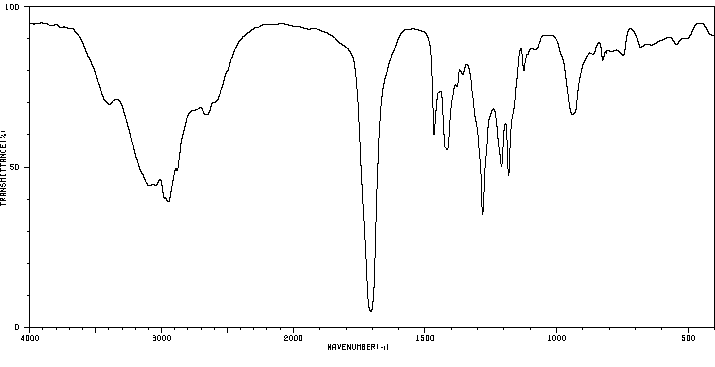
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息