4-甲基-异噻唑 | 693-90-3
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:147 °C
-
密度:1.0187 g/cm3
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:6
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:41.1
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2934999090
SDS
反应信息
-
作为反应物:参考文献:名称:Phototransposition chemistry of methylisothiazoles and methylthiazoles摘要:Methylisothiazoles undergo phototransposition in neutral solution to methylthiazoles by a single permutation process. Methylisothiazole --> methylisothiazole transpositions, previously reported to occur, were not detected in these reactions. In trifluoroacetic acid solvent, protonated methylisothiazoles and methylthiazoles phototranspose by P4 and P5 permutation pathways, respectively, resulting in a unique phototransposition cycle.DOI:10.1021/jo00064a033
-
作为产物:描述:参考文献:名称:Phototransposition Chemistry of 4-Substituted Isothiazoles. The P4 Permutation Pathway摘要:Upon irradiation in the presence of a small quantity of base, 4-substituted isothiazoles undergo photocleavage to yield substituted cyanosulfides, which can be trapped as their benzyl thioether derivatives, and substituted isocyanosulfides. Both products are suggested to arise via initial photocleavage of the sulfur-nitrogen bond, resulting in the formation of a substituted beta-thioformylvinyl nitrene, which can rearrange to the observed cyanosulfide, or cyclize to an undetected thioformylazirine. Deprotonation of the azirine leads directly to the isocyanosulfide. The plight of the isocyanosulfide depends on the C-4 substituent. If the substituent is aromatic, the isocyanosulfide is reprotonated at the isocyanide carbon and spontaneously cyclizes to a 4-substituted thiazole, the observed transposition product. If the substituent is not aromatic, the isocyanosulfide is reprotonated at sulfur and the resulting species has a higher energy barrier to cyclization. In these cases, the isocyanosulfides can be observed spectroscopically and can be trapped as their N-formylaminobenzyl thioether derivatives.DOI:10.1021/jo980936e
文献信息
-
Lithiation of five-membered heteroaromatic compounds. The methyl substituted 1,2-azoles, oxadiazoles, and thiadiazoles作者:R. G. MicetichDOI:10.1139/v70-334日期:1970.7.1
The lithiation of various methyl substituted isoxazoles, isothiazoles, pyrazoles, oxadiazoles, and thiadiazoles using n-butyllithium has been studied. Three types of reactions, namely, lateral lithiation, ring cleavage, and addition of butyllithium to the ring, have been found. 3,5-Dimethylisoxazole, 3-phenyl-5-methylisoxazole, 3,4-dimethyl-1,2,5-oxadiazole, 2,5-dimethyl-1,3,4-thiadiazole, 3-phenyl-5-methyl-1,2,4-oxadiazole, and 3,5-dimethyl-1,2,4-thiadiazole all undergo lateral lithiation to give the respective acetic acids after carboxylation. 1-Methyl-3,5-disubstituted pyrazoles form the 1-lithiomethyl derivatives, while 1-phenyl-3,5-disubstituted pyrazoles are converted to the 1-ortholithiophenyl-3,5-disubstituted pyrazoles. 4-Methylisothiazole is lithiated mainly at C-5, but also suffers ring cleavage to form 1-n-butylthio-2-cyanoprop-1-ene. Heteroaromatic compounds containing an N—S bond, such as 3,4-dimeth yl-1,2,5-thiadiazole, 4-methyl-5-phenyl-1,2,3-thiadiazole, and 3,5-dimethylisothiazole, undergo nucleophilic attack at sulfur with resulting ring cleavage. 3,5-Dimethylisothiazole produces 2-n-butylthiopent-2-en-4-one. 3-Methyl-5-phenyl-1,2,4-oxadiazole gave 3-methyl-5-phenyl-5-n-butyl-1,2,4-dihydroöxadiazole by addition to the azomethine bond. The results of these lithiations are discussed. 3-Methyl-5-lithiomethylisoxazole was converted to various derivatives. Nuclear magnetic resonance spectral analysis was used to establish the identity of the products.
各种甲基取代异噁唑、异硫唑、吡唑、噁二唑和噻二唑的锂化反应已经被研究。发现了三种类型的反应,即侧链锂化、环裂解和丁基锂加入环中。3,5-二甲基异噁唑、3-苯基-5-甲基异噁唑、3,4-二甲基-1,2,5-噁二唑、2,5-二甲基-1,3,4-噻二唑、3-苯基-5-甲基-1,2,4-噁二唑和3,5-二甲基-1,2,4-噻二唑都经历侧链锂化,在羧化后生成相应的乙酸。1-甲基-3,5-二取代吡唑形成1-锂甲基衍生物,而1-苯基-3,5-二取代吡唑转化为1-邻锂苯基-3,5-二取代吡唑。4-甲基异硫唑主要在C-5处发生锂化,但也发生环裂解形成1-正丁硫基-2-氰基丙-1-烯。含有N—S键的杂环化合物,如3,4-二甲基-1,2,5-噻二唑、4-甲基-5-苯基-1,2,3-噻二唑和3,5-二甲基异硫唑,在硫原子上发生亲核攻击,导致环裂解。3,5-二甲基异硫唑产生2-正丁硫代戊-2-烯-4-酮。3-甲基-5-苯基-1,2,4-噁二唑通过加成到偶氮亚胺键生成3-甲基-5-苯基-5-正丁基-1,2,4-二氢噁二唑。讨论了这些锂化的结果。3-甲基-5-锂甲基异噁唑被转化为各种衍生物。核磁共振光谱分析用于确定产物的身份。 -
SYNTHESES AND ANTIBACTERIAL ACTIVITIES OF PENICILLINS FROM (+)- AND (-)-a-AMINO- 4-ISOTHIAZOLYLACETIC ACIDS作者:R. RAAPDOI:10.7164/antibiotics.24.695日期:——α-Amino-4-isothiazolylacetic acid has been prepared by reaction of α-bromo-4-isothiazalylacetic acid with ammonium hydroxide and by catalytic hydrogenation of α-azido-4-isothiazolylacetic acid. The α-azido acid has been resolved into its two optical isomers from which the optically active amino acids were prepared. The absolute configurations of these amino acids are tentatively assigned. The dextrorotatory isomer can also be prepared directly by resolution of the racemic amino acid with d-camphor-10-sulfonic acid. From the optically active amino acids the penicillins were synthesized by the activated ester method using the p-nitrocarbobenzoxy protecting group. Some MIC and CD50 values against Gram-positive and Gram-negative bacteria are given. The introduction of an α-amino group into 4-isothiazolylmethylpenicillin produces only a minimal effect in the Gram-negative activity.
-
[EN] HETEROARYL-SUBSTITUTED MACROCYCLIC FLU ENDONUCLEASE INHIBITORS<br/>[FR] INHIBITEURS MACROCYCLIQUES D'ENDONUCLÉASE DE LA GRIPPE SUBSTITUÉS PAR HÉTÉROARYLE申请人:JANSSEN BIOPHARMA INC公开号:WO2021191872A1公开(公告)日:2021-09-30The present invention relates to heteroaryl-substituted macrocyclic compounds and prodrugs thereof, and pharmaceutically acceptable salts, solvates, and polymorphs thereof, and the use of such compounds as a medicament, in particular in the prevention and/or treatment of viral infections caused by viruses belonging to the Orthomyxoviridae family. The present invention furthermore relates to pharmaceutical compositions or combination preparations of the compounds, and to the compositions or preparations for use as a medicament, more preferably for the prevention or treatment of viral infections caused by viruses belonging to the Orthomyxoviridae family.本发明涉及杂环取代的大环化合物及其前药,以及其药学上可接受的盐、溶剂化合物和多型体,以及将这些化合物作为药物的用途,特别是用于预防和/或治疗由属于Orthomyxoviridae家族的病毒引起的病毒感染。本发明还涉及该化合物的药物组合物或混合制剂,以及用作药物的组合物或制剂,更好地用于预防或治疗由属于Orthomyxoviridae家族的病毒引起的病毒感染。
-
Five-membered Heterocyclic Thiones. Part V.The Sulfurization of 5-Lithioisothiazoles作者:Donald E. Horning、Joseph M. MuchowskiDOI:10.1139/v74-431日期:1974.8.15
5-Lithioisothiazoles reacted with sulfur at −70° to produce the corresponding unstable (in air) lithium isothiazole-5-thiolates. The salts could be utilized insitu to prepare 5-alkylthioisothiazoles or they could be transformed in low yield into stable mercuric thiolates.
-
Heterocyclic analogs of chlorcyclizine with potent hypolipidemic activity作者:Michael J. Ashton、Alan Ashford、Anthony H. Loveless、David Riddell、John Salmon、Gregory V. W. StevensonDOI:10.1021/jm00376a002日期:1984.10A series of [alpha-(heterocyclyl)benzyl]piperazines was synthesized and their effect of reducing serum cholesterol and triglyceride levels in the rat was evaluated. A systematic exploration of the structure-activity relationships led to the synthesis of (R,S)-(3,5-dimethylisoxazol-4-yl)[4-(1-methylethyl)phenyl] (4-methylpiperazin-1-yl)methane dihydrochloride (M&B 31 426), which had potent activity in lowering serum lipid levels at a daily oral dose of 2 mg/kg and was 100 times more potent than clofibrate.
表征谱图
-
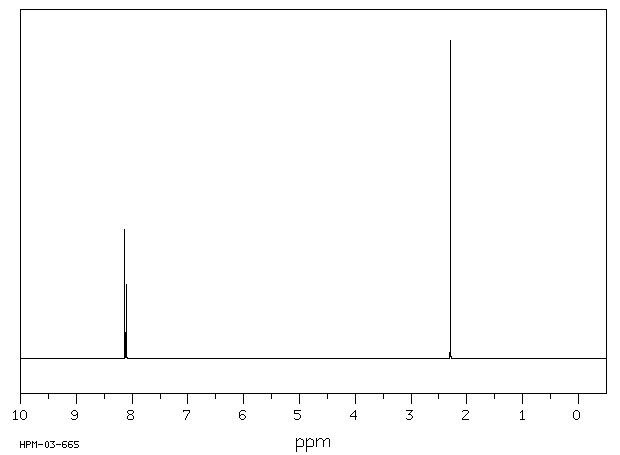
氢谱1HNMR
-
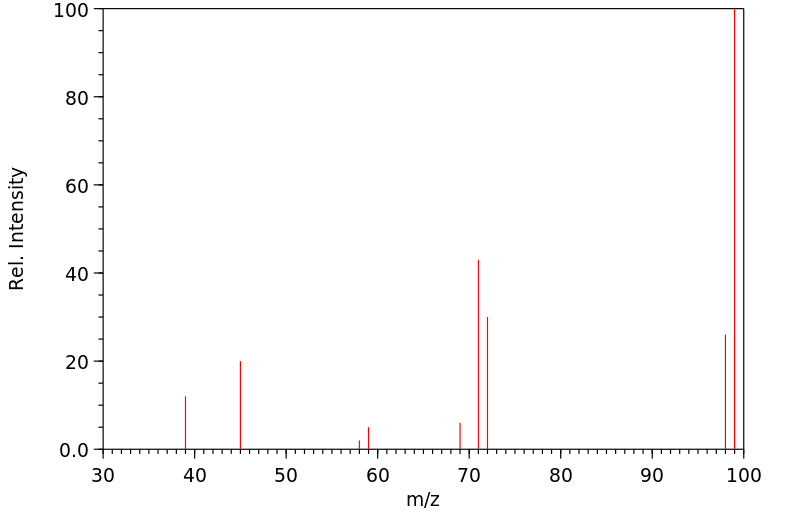
质谱MS
-
碳谱13CNMR
-
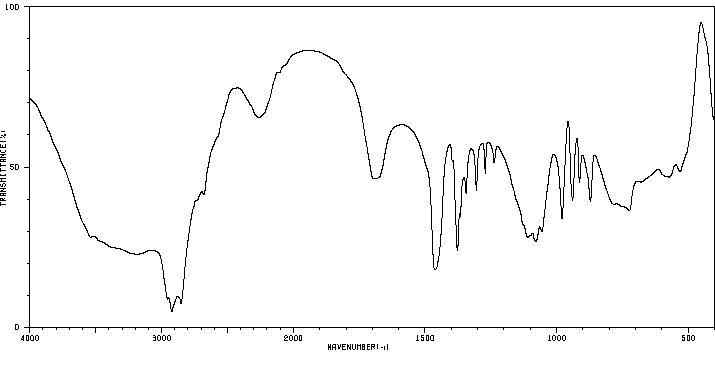
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息