1-苯基-1-环己羧酸 | 1135-67-7
中文名称
1-苯基-1-环己羧酸
中文别名
1-苯基环己烷羧酸
英文名称
1-phenyl-cyclohexanecarboxylic acid
英文别名
1-phenylcyclohexane-1-carboxylic acid;1-phenyl-1-cyclohexanecarboxylic acid;1-phenyl-1-cyclohexyl carboxylic acid;1-Phenylcyclohexanecarboxylic acid
CAS
1135-67-7
化学式
C13H16O2
mdl
——
分子量
204.269
InChiKey
QXXHHHWXFHPNOS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:119-124 °C
-
沸点:302.76°C (rough estimate)
-
密度:1.0404 (rough estimate)
-
溶解度:溶于甲醇
-
稳定性/保质期:
如果按照规格正确使用和储存,则不会发生分解,没有已知的危险反应。请避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:15
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.46
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
安全说明:S24/25
-
海关编码:2916399090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:请将贮藏器密封,并存放在阴凉、干燥处。确保工作环境具有良好的通风或排气设施。
SDS
| Name: | 1-Phenyl-1-cyclohexanecarboxylic acid 95% Material Safety Data Sheet |
| Synonym: | 1-Phenylcyclohexane-1-carboxylic aci |
| CAS: | 1135-67-7 |
Synonym:1-Phenylcyclohexane-1-carboxylic aci
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1135-67-7 | 1-Phenyl-1-cyclohexanecarboxylic acid | 95 | 214-495-8 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Avoid contact with skin and eyes. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1135-67-7: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: beige - brown
Odor: None reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 121 - 123 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: Not available.
Specific Gravity/Density: Not available.
Molecular Formula: C13H16O2
Molecular Weight: 204.27
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1135-67-7 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1-Phenyl-1-cyclohexanecarboxylic acid - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 1135-67-7: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1135-67-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1135-67-7 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
聚合物和大分子半导体砌块
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-苯基-1-环己烷羧酸甲酯 methyl 1-phenylcyclohexanecarboxylate 17380-78-8 C14H18O2 218.296 (1-苯基环己烷)甲醇 (1-phenylcyclohexyl)methanol 68692-77-3 C13H18O 190.285 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-苯基-1-环己烷羧酸甲酯 methyl 1-phenylcyclohexanecarboxylate 17380-78-8 C14H18O2 218.296 —— 1-[4-(methoxycarbonyl)phenyl]cyclohexanecarboxylic acid 732308-74-6 C15H18O4 262.306 1-(4-硝基苯基)环己烷羧酸 1-(4-nitro-phenyl)-cyclohexanecarboxylic acid 91958-27-9 C13H15NO4 249.266 —— 1-Phenyl-cyclohexanecarboperoxoic acid tert-butyl ester 68580-52-9 C17H24O3 276.376 (1-苯基环己烷)甲醇 (1-phenylcyclohexyl)methanol 68692-77-3 C13H18O 190.285 1-苯基环己烷-1-甲醛 1-phenylcyclohexanecarbaldehyde 22612-69-7 C13H16O 188.269 —— UCB 597 13877-96-8 C21H33NO3 347.498 —— 1-Phenylcyclohexylmethylketon 3183-57-1 C14H18O 202.296 —— 1-<1-Phenyl-cyclohexyl>-propan-1-on 2886-61-5 C15H20O 216.323 —— Isopropyl-<1-phenyl-cyclohexyl>-keton 2891-54-5 C16H22O 230.35 1-苯基环己烷羰酰氯 1-phenylcyclohexylcarbonyl chloride 2890-42-8 C13H15ClO 222.715 1-苯基环己烷-1-甲酰胺 1-phenylcyclohexane-1-carboxamide 2890-60-0 C13H17NO 203.284 —— O-<2-<2-(diethylamino)ethoxy>ethyl>-1-phenyl-1-cyclohexanemethanol 136089-16-2 C21H35NO2 333.514 —— 2H-spiro[benzofuran-3,1'-cyclohexan]-2-one 1396689-98-7 C13H14O2 202.253 —— 2-Diazo-1-(1-phenyl-cyclohexyl)-ethanone 110397-48-3 C14H16N2O 228.294 - 1
- 2
反应信息
-
作为反应物:描述:1-苯基-1-环己羧酸 在 lithium aluminium tetrahydride 、 叠氮磷酸二苯酯 、 氧气 、 copper diacetate 、 magnesium sulfate 、 三乙胺 作用下, 以 四氢呋喃 、 甲苯 为溶剂, 150.0 ℃ 、101.33 kPa 条件下, 反应 36.0h, 生成 2,3,4,9-四氢-9-甲基-1H-咔唑参考文献:名称:通过氮-自由基4-exo-trig环化反应进行铜催化的有氧[1,3]-氮转移摘要:在氧气氛(1个大气压)下,由二乙酸铜催化的新型[1,3]-氮自由基转化已被开发出来,用于从α,α-二取代的苄胺中构建各种吲哚衍生物。在该反应中,氧气用作清洁的终端氧化剂,而水是唯一的副产物。在该转化过程中,五个惰性键被裂解,并且在一个罐中构建了两个C-N键和一个C-C双键。这种独特的方法证明了其对生物活性天然产物和药物的后期修饰的广泛应用潜力。机理研究表明,氨基自由基在苯环上的独特的4- exo- trig环化涉及催化循环。DOI:10.1002/anie.201709894
-
作为产物:描述:参考文献:名称:异羟肟酸酯AR-42的手性衍生物有效抑制I类HDAC酶和癌细胞的增殖。摘要:在多发性骨髓瘤,白血病和淋巴瘤的临床试验中,AR-42是组蛋白脱乙酰基酶(HDAC)的口服活性抑制剂。它几乎没有氢键供体和受体,但是是手性的2-芳基丁酸酯,可能易于消旋。我们报道了非手性AR-42类似物,其包含通过季碳原子连接的环烷基,对人类I类HDAC的效力提高了40倍(例如,JT86,IC 500.7 nM,HDAC1),对五种人类癌细胞系的细胞毒性增加了25倍,而对正常人类细胞的毒性降低了多达70倍。JT86在促进MM96L黑色素瘤细胞中乙酰化组蛋白H4积累方面比racAR-42强9倍。分子模型和结构-活性之间的关系支持四氢吡喃与HDAC1的结合,而四氢吡喃则是对酶表面水的疏水屏蔽。这种有效的I类HDAC抑制剂可在AR-42活跃的疾病(癌症,寄生虫感染,炎性疾病)中显示出益处。DOI:10.1021/acs.jmedchem.0c00230
-
作为试剂:描述:4-甲氧基肉桂醛 、 (5E)-7-oxo-7-phenyl-hept-5-enal 在 1-(4-((R)-(naphthalen-1-ylmethoxy)((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl)methyl)quinolin-6-yl)-3-phenylthiourea 、 D-脯氨酸 、 1-苯基-1-环己羧酸 作用下, 以 甲苯 为溶剂, 反应 72.08h, 以45%的产率得到参考文献:名称:可切换模块化设计的有机催化剂催化的多米诺迈克尔/迈克尔反应摘要:通过使用模块化设计的有机催化剂 (MDO) 研究了( E )-7-aryl-7-oxohept-5-enals 和反式肉桂醛之间的多米诺 Michael/Michael 反应。发现 MDO 的烯胺和亚胺催化模式都是可切换的,并且可以通过使用预催化剂模块和反应条件的适当组合来单独打开和关闭。当开启 MDO 的烯胺和亚胺催化模式时,可以在优化的条件下以良好的收率和立体选择性获得所需的多米诺反应产物。DOI:10.1039/d1ob01991k
文献信息
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
COMPOSITIONS FOR TREATMENT OF CYSTIC FIBROSIS AND OTHER CHRONIC DISEASES申请人:Van Goor Fredrick F.公开号:US20110098311A1公开(公告)日:2011-04-28The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
Oxidation of Primary Alcohols and Aldehydes to Carboxylic Acids via Hydrogen Atom Transfer作者:Wen-Yun Tan、Yi Lu、Jing-Feng Zhao、Wen Chen、Hongbin ZhangDOI:10.1021/acs.orglett.1c02188日期:2021.9.3The oxidation of primary alcohols and aldehydes to the corresponding carboxylic acids is a fundamental reaction in organic synthesis. In this paper, we report a new chemoselective process for the oxidation of primary alcohols and aldehydes. This metal-free reaction features a new oxidant, an easy to handle procedure, high isolated yields, and good to excellent functional group tolerance even in the
-
NOVEL COMPOUNDS AS CANNABINOID RECEPTOR LIGANDS申请人:Carroll William A.公开号:US20090018114A1公开(公告)日:2009-01-15The present application relates to cannabinoid receptor ligands containing compounds of formula (I) wherein A, R 1 , R 2 , and R 3 are as defined in the specification. The present application also relates to compositions comprising such compounds, and methods of treating conditions and disorders using such compounds and compositions.
-
NOVEL SUBSTITUTED OCTAHYDROCYCLOPENTA[C]PYRROL-4-AMINES AS CALCIUM CHANNEL BLOCKERS申请人:Stewart Andrew O.公开号:US20100130558A1公开(公告)日:2010-05-27The present application relates to calcium channel inhibitors containing compounds of formula (I) wherein L 1 , L 2 , R 1 , R 2 , and R 3 are as defined in the specification. The present application also relates to compositions comprising such compounds, and methods of treating conditions and disorders using such compounds and compositions.
表征谱图
-
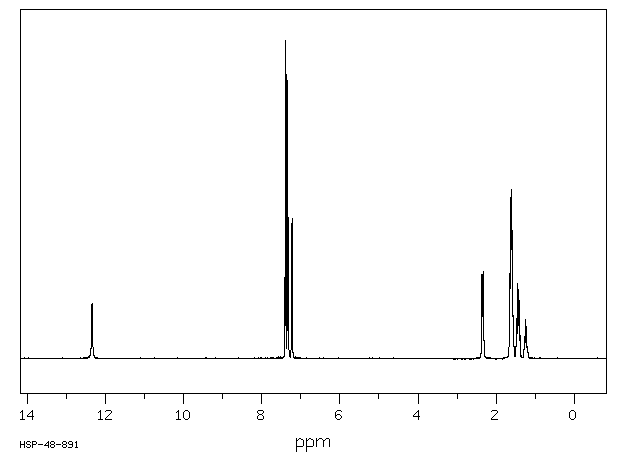
氢谱1HNMR
-
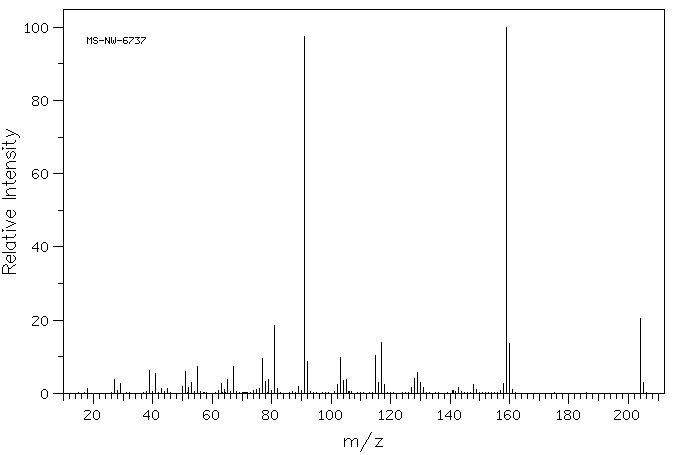
质谱MS
-
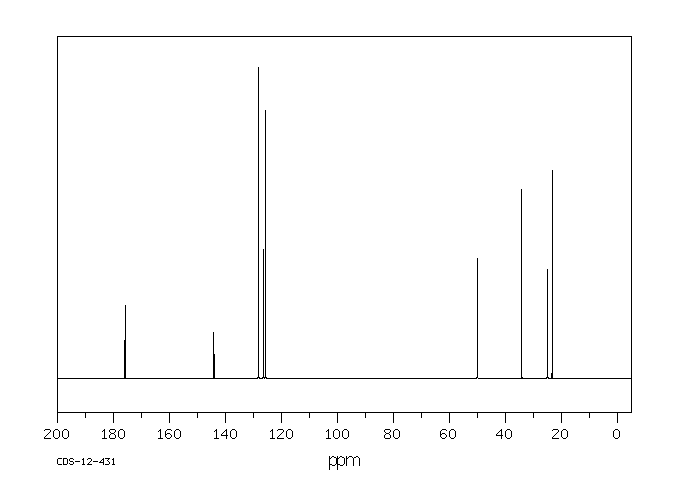
碳谱13CNMR
-
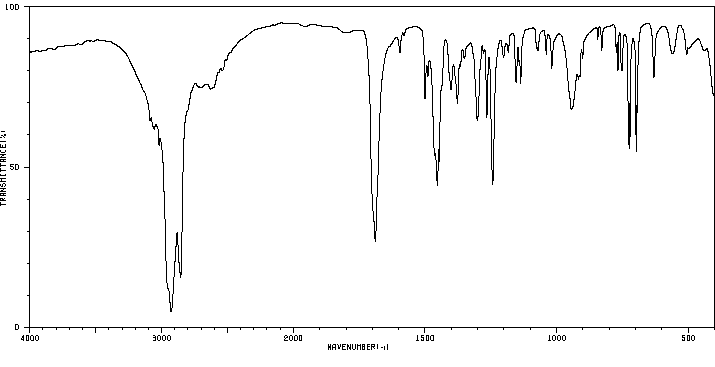
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫