抑芽丹 | 123-33-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:299-301 °C (dec.)(lit.)
-
沸点:209.98°C (rough estimate)
-
密度:1,6 g/cm3
-
闪点:300°C
-
溶解度:4水中的溶解度为510毫克/升
-
物理描述:Maleic hydrazide is an odorless white solid. Sinks in water. (USCG, 1999)
-
颜色/状态:Crystals from water
-
气味:Odorless
-
蒸汽压力:7.5X10-8 mm Hg at 25 °C
-
亨利常数:Henry's Law constant = 8.1X10-9 atm-cu m/mol at 25 °C and pH 7
-
稳定性/保质期:
有刺激性物质,会对眼睛、呼吸道和皮肤产生刺激作用。请穿戴适当的防护服、手套,并佩戴防护眼镜或面罩。
-
分解:DT50 (25 °C) 58 days (pH 5, 7), 34 days (pH 9).
-
腐蚀性:Liquid formulated products are corrosive to brass nozzles if sprayed undiluted.
-
燃烧热:-8,200 BTU/LB= -4,500 CAL/G= -190X10+5 J/KG (EST)
-
解离常数:pKa = 5.62 at 20 °C
计算性质
-
辛醇/水分配系数(LogP):-0.8
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:58.2
-
氢给体数:2
-
氢受体数:2
ADMET
安全信息
-
TSCA:Yes
-
危险品标志:Xn,Xi
-
安全说明:S26,S36,S36/37/39,S45
-
危险类别码:R68,R40,R36/37/38
-
WGK Germany:2
-
海关编码:2928000090
-
危险品运输编号:UN 3077 9/PG 3
-
RTECS号:UR5950000
-
危险标志:GHS07,GHS08
-
危险性描述:H315,H319,H335,H341
-
危险性防范说明:P261,P281,P305 + P351 + P338
-
储存条件:库房应保持通风、低温和干燥,并与其他食品原料分开储运。
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 马来酰肼,顺丁烯二酰肼 |
| 化学品英文名称: | Maleichydrazine;1,2-Dihydro-3,6-pyridiazinediOne |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 123-33-1 |
| 分子式: | C 4 H 4 N 2 O 2 |
| 分子量: | 112.10 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | |||
| 化学品名称:马来酰肼,顺丁烯二酰肼 | |||
|
| 第三部分:危险性概述 |
| 危险性类别: | |
| 侵入途径: | 吸入 食入 |
| 健康危害: | 动物中毒表现呈抑郁状态,呼吸增快,肢端紫绀,并见震颤、痉挛和瘫痪 |
| 环境危害: | |
| 燃爆危险: |
| 第四部分:急救措施 |
| 皮肤接触: | 用肥皂水及清水彻底冲洗。就医。 |
| 眼睛接触: | 拉开眼睑,用流动清水冲洗15分钟。就医。 |
| 吸入: | 脱离现场至空气新鲜处。就医。 |
| 食入: | 误服者,饮适量温水,催吐。就医。 |
| 第五部分:消防措施 |
| 危险特性: | 遇明火、高热可燃。受高热分解放出有毒的气体。 |
| 有害燃烧产物: | |
| 灭火方法及灭火剂: | 雾状水、抗溶性泡沫、二氧化碳、干粉、水。 |
| 消防员的个体防护: | |
| 禁止使用的灭火剂: | |
| 闪点(℃): | |
| 自燃温度(℃): | |
| 爆炸下限[%(V/V)]: | |
| 爆炸上限[%(V/V)]: | |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 隔离泄漏污染区,周围设警告标志,建议应急处理人员戴好口罩、护目镜,穿工作服。不要直接接触泄漏物,小心扫起,避免扬尘,运至废物处理场所。也可以用大量水冲洗,经稀释的污水放入废水系统。如大量泄漏,收集回收或无害处理后废弃。 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | |
| 储存注意事项: |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 中 国 MAC:未制订标准前苏联 MAC:未制订标准美国TLV—TWA:未制订标准 |
| 监测方法: | |
| 工程控制: | 密闭操作,局部排风。 |
| 呼吸系统防护: | 作业工人建议佩戴防尘口罩。 |
| 眼睛防护: | 可采用安全面罩。 |
| 身体防护: | 穿相应的防护服。 |
| 手防护: | 戴防护手套。 |
| 其他防护: | 工作现场禁止吸烟、进食和饮水。工作后,淋裕更衣.保持良好的卫生习惯。 |
| 第九部分:理化特性 |
| 外观与性状: | 白色结晶体。 |
| pH: | |
| 熔点(℃): | 296~298(分解) |
| 沸点(℃): | |
| 相对密度(水=1): | 1.60(25℃) |
| 相对蒸气密度(空气=1): | |
| 饱和蒸气压(kPa): | |
| 燃烧热(kJ/mol): | |
| 临界温度(℃): | |
| 临界压力(MPa): | |
| 辛醇/水分配系数的对数值: | |
| 闪点(℃): | |
| 引燃温度(℃): | |
| 爆炸上限%(V/V): | |
| 爆炸下限%(V/V): | |
| 分子式: | C 4 H 4 N 2 O 2 |
| 分子量: | 112.10 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 微溶于冷水,溶于热水、热碱液,不溶于醚、氯仿。 |
| 主要用途: | 用于防止禾谷、马玲薯、洋葱等在贮存期发芽变质,也可用作除草剂。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 稳定 |
| 禁配物: | 强氧化剂、强酸。 |
| 避免接触的条件: | |
| 聚合危害: | 不能出现 |
| 分解产物: | 一氧化碳、二氧化碳、氮氧化物。 |
| 第十一部分:毒理学资料 |
| 急性毒性: | LD50:4000mg/kg(大鼠经口) LC50: |
| 急性中毒: | |
| 慢性中毒: | |
| 亚急性和慢性毒性: | |
| 刺激性: | |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | |
| 生物降解性: | |
| 非生物降解性: | |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | |
| UN编号: | |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | |
| 运输注意事项: | 储存于阴凉、通风仓间内。远离火种、热源。包装密封。防止受潮和雨淋。防止阳光曝晒。应与氧化剂、酸类、食用化工原料分开存放。操作现场不得吸烟、饮水、进食。搬运时轻装轻卸,保持包装完整,防止洒漏。分装和搬运 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | 1.周国泰,化学危险品安全技术全书,化学工业出版社,1997 2.国家环保局有毒化学品管理办公室、北京化工研究院合编,化学品毒性法规环境数据手册,中国环境科学出版社.1992 3.Canadian Centre for Occupational Health and Safety,CHEMINFO Database.1998 4.Canadian Centre for Occupational Health and Safety, RTECS Database, 1989 |
| 填表时间: | 年月日 |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | 6 |
| MSDS修改日期: | 年月日 |
制备方法与用途
毒性 大鼠急性经口LD₅₀为1400 mg/kg,其钠盐为6950 mg/kg,二乙醇盐为2340 mg/kg。无刺激作用。用含5%原药的饲料喂养大鼠两年未出现中毒症状,未见致畸、致癌、致突变性。对鱼低毒,鲈鱼LC₅₀为75 mg/L。对蜜蜂无毒。
化学性质 纯品为白色结晶固体,熔点(m.p.)296~298℃,相对密度1.60 (25℃),闪点300℃,不易挥发。25℃时溶解度:二甲基甲酰胺2.4%,丙酮<1%,二甲苯<1%,乙醇<0.1%,水0.6%。性质稳定,遇强酸可分解放出氮,对铁器有轻微腐蚀性。原药纯度>97%,m.p. >292℃,相对密度1.60。其三乙醇胺盐在水中溶解度为70%,钾盐10%,钠盐20%。
用途 植物生长调节剂。通过叶面或根部吸入,由木质部和韧皮部传导,抑制细胞分裂而抑制植物生长。0.1%-0.05%药液可防除禾草和草坪、果园及水边杂草;0.025%药液抑制洋葱和马铃薯贮存期发芽;0.1%-0.05%药液抑制及延缓烟草侧芽生长和保持柑橘苗免受霜害。
顺丁烯二酰肼为选择性除草剂和暂时性植物生长抑制剂。药剂可通过叶面角质层进入植株,降低光合作用、渗透压和蒸发作用,能强烈地抑制芽的生长。用于防止土豆、洋葱、大蒜和萝卜贮藏期发芽,并有抑制作物生和延长开花的作用,也可用于非耕地除草。该品的互变异构为3,6-二羟基哒嗪(3,6-Dihydroxypyridazine)。是磺胺类药物磺胺甲氧嗪的中间体。
用途 用作农药、医药中间体。 用途 用作生化研究。
生产方法 由顺丁烯二酸酐与水合肼反应而得。在反应锅中投入水及40%水合肼,搅拌冷却下滴加30%盐酸,温度控制在20℃以下,至pH值为6.2-6.4,投入顺丁烯二酸酐,缓缓升温至106-110℃,回流2小时,降温至5℃,甩滤,滤饼用冰水洗至pH4.8-5.1,干燥,得顺丁烯二酰肼,收率97%。
生产方法 由丁烯二酸酐(或丁二烯二酸)与硫酸肼于35~56℃下反应制得。
类别 农药
毒性分级 中毒
急性毒性 口服- 大鼠 LD₅₀:3000 毫克/公斤
可燃性危险特性 燃烧产生有毒氮氧化物气体
储运特性 库房通风低温干燥;与食品原料分开储运
灭火剂 干粉、泡沫、砂土
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3,6-二甲氧基吡嗪 3,6-dimethoxypyridazine 4603-59-2 C6H8N2O2 140.142
反应信息
-
作为反应物:描述:参考文献:名称:Synthesis of New Derivatives of Pyridazino[6,1-c]Pyrimido[5,4-e][1,2,4]Triazine; a Novel Heterocyclic System摘要:通过在沸腾的乙醇中将 5-溴-2,4-二氯-6-甲基嘧啶与 3-氯-6-肼基哒嗪杂环化,然后用仲胺处理,合成了几种新型哒嗪并[6,1-c]嘧啶并[5,4-e][1,2,4]三嗪环系统的衍生物。DOI:10.3184/174751916x14497690510968
-
作为产物:参考文献:名称:利用 azothiacalix[4] 芳烃官能化羧甲基纤维素聚合物配体进行铜 (II) 离子检测的简便化学发光策略摘要:目前工作的主要目的是开发一种基于鲁米诺的化学发光 (CL) 系统,该系统借助氮芥[4]芳烃改性羧甲基纤维素 (ACCMC) 的反应性功能聚合物用于铜 ( II ) 离子的痕量分析. ACCMC首次通过[2+3]偶极环加成点击反应将水溶性脱氧叠氮基羧甲基纤维素与单炔丙基-四氮杂杯[4]芳烃连接合成。所得材料通过 FT-IR、1 H 和13表征C NMR 光谱、TGA 和 FE-SEM。在最佳条件下,AC 和 ACCMC 在碱性溶液中通过过氧化氢辅助氧化来猝灭鲁米诺和 1,2-二氢-3,6-哒嗪二酮的 CL 强度,并评估其对铜 ( II ) 离子的检测Cu( II )的增强作用。根据Cu 2+存在下的CL光谱,鲁米诺-H 2 O 2、鲁米诺-AC-H 2 O 2和鲁米诺-ACCMC-H 2 O 2的发光强度分别增加到约 2、6.5 和 3 倍。引入的系统提供宽范围的发光,具有在 CL 系统中分析或测量分析物的优势。制备的DOI:10.1039/d2nj00451h
-
作为试剂:参考文献:名称:氢键对具有刚性酰胺间隔基的二茂铁-醌二元组中金属离子促进的分子内电子转移和光致电子转移的影响摘要:具有刚性酰胺间隔基的二茂铁-醌二元组 (Fc-Q) 和 Fc-(Me)Q 二元组,其中作为氢键受体的酰胺质子被甲基取代,用于检查热和光致电子转移反应中的氢键。Fc-Q 的电子转移还原产生的半醌自由基阴离子与 Fc-Q(.-) 中酰胺质子的氢键合由 Fc-Q 的单电子还原电位的显着正移表示。Fc-Q(.-) 的超精细耦合常数也表明存在氢键,与密度泛函计算预测的一致。在飞秒激光闪光光解实验中,已成功检测到从二茂铁 (Fc) 到 Fc-Q 中醌部分 (Q) 的光致电子转移中的氢键动力学。在 298 K 的乙腈中存在金属离子时,Fc-Q 和 Fc-(Me)Q 中从 Fc 到 Q 的分子内电子热转移也有效发生。半醌自由基阴离子与 Fc-中的酰胺质子之间形成氢键与禁止氢键的 Fc-(Me)Q 的速率相比,Q 导致金属离子促进电子转移的速率显着加快。金属离子促进的电子转移速率与超氧离子-金属离子复合物的结合能密切相关,这些结合能来自DOI:10.1021/ja026441v
文献信息
-
Cytotoxic ring A-modified steroid analogues derived from Grundmann’s ketone作者:Christoph D. Mayer、Franz BracherDOI:10.1016/j.ejmech.2011.04.036日期:2011.8A series of steroid and azasteroid analogues containing a six-membered ring A with various functionalities were synthesized. Furthermore, the syntheses of tetracyclic analogues bearing a five-membered A-ring and the syntheses of a number of bicyclic secosteroid analogues were carried out. All compounds were tested for their antibacterial, antifungal and cytotoxic activities. Among all tested compounds
-
Benzopyranes as potassium channel openers申请人:Pfizer Inc.公开号:US05677324A1公开(公告)日:1997-10-14The present invention relates to compounds of formula (I) and the pharmaceutically acceptable salts thereof, wherein the dashed line represents an optional covalent bond; X is O, NH, S or a direct link; R.sup.3 is hydroxy when the dashed line does not represent a covalent bond and R.sup.3 is absent when the dashed line represents a covalent bond; R.sup.4 is (a), when X is O, a group of formula (i), (b), when X is O, NH or S, optionally substituted hydroxyphenyl, (c) an optionally substituted 4- to 7-membered heterocyclic ring, or (d), when X is NH, a group of formula (ii). The compounds are useful for the treatment of disease associated with the altered tone or motility of smooth muscle. ##STR1##本发明涉及式(I)的化合物及其药学上可接受的盐,其中虚线代表可选的共价键;X为O、NH、S或直接连接;当虚线不代表共价键时,R.sup.3为羟基,当虚线代表共价键时,R.sup.3不存在;当X为O时,R.sup.4为(a),为式(i)的基团,当X为O、NH或S时,为可选取代的羟基苯基(b),为可选取代的4-至7-成员杂环环(c),或当X为NH时,为式(ii)的基团(d)。这些化合物对于治疗与平滑肌张力或运动异常相关的疾病是有用的。
-
Efficient Phosphorus-Free Chlorination of Hydroxy Aza-Arenes and Their Application in One-Pot Pharmaceutical Synthesis作者:Jian Wang、Yan-Hui Li、Song-Cheng Pan、Ming-Fang Li、Wenting Du、Hong Yin、Jing-Hua LiDOI:10.1021/acs.oprd.9b00407日期:2020.2.21The chlorination of hydroxy aza-arenes with bis(trichloromethyl) carbonate (BTC) and SOCl2 has been effectively performed by refluxing with 5 wt % 4-dimethylaminopyridine (DMAP) as a catalyst. Various substrates are chlorinated with high yields. The obtained chlorinated aza-arenes can be used directly with simple workup for succedent one-pot synthesis on a large scale.
-
Pyridazinones. 1. Synthesis, antisecretory, and antiulcer activities of thio amide derivatives作者:Toshihiro Yamada、Youichi Nobuhara、Azuma Yamaguchi、Masahiko OhkiDOI:10.1021/jm00350a018日期:1982.83(2H)-pyridazinone derivatives and related analogues was synthesized. Substituted 3(2H)-pyridazinones and their 4,5-dihydro analogues were alkylated by omega-haloalkyl cyanides at the N-2 position under phase-transfer catalytic reaction, and the nitrile group was converted to the thio amide group by treatment with hydrogen sulfide alone or with the appropriate primary or secondary amines. Various substituents为了开发新型抗溃疡药,合成了一系列新型3(2H)-哒嗪酮衍生物和相关类似物。在相转移催化反应下,在N-2位用ω-卤代烷基氰化物将取代的3(2H)-吡啶并壬酮及其4,5-二氢类似物烷基化,并通过氢处理将腈基转化为硫代酰胺基。硫化物单独使用或与适当的伯胺或仲胺一起硫化。在硫代酰胺的氮原子,侧链的碳原子和3(2H)-哒嗪酮环上引入了各种取代基。评价合成的化合物在幽门结扎的大鼠中胃的抗分泌活性,并将选择的化合物应用于实验性溃疡模型,例如大鼠的Shay's,阿司匹林诱导和应激诱导的溃疡。讨论了构效关系。在测试的化合物中,具有C-6苯基和带有末端硫代酰胺基团(48、49、51和52)的N-2烷基侧链的3(2H)-吡嗪酮是最有效的。
-
Novel pyrazoles and pyrazolo[1,2- a ]pyridazines as selective COX-2 inhibitors; Ultrasound-assisted synthesis, biological evaluation, and DFT calculations作者:Nagat Ghareb、Hosam A. Elshihawy、Mohamed M. Abdel-Daim、Mohamed A. HelalDOI:10.1016/j.bmcl.2017.04.020日期:2017.6devoid of ulcerogenic activity. Herein, we report the design and synthesis of a series of pyrazoles and pyrazolo[1,2-a]pyridazines with selective COX-2 inhibitory activity and in vivo anti-inflammatory effect. Both series were accessed through acid-catalyzed ultrasound-assisted reactions. The most active compounds in this study are two novel molecules, 11 and 16, showing promising selectivity and decentCOX-2是一种介导炎症反应的诱导酶。COX-2的选择性靶向可用于开发无溃疡活性的抗炎药。在这里,我们报告设计和合成一系列具有选择性COX-2抑制活性和体内抗炎作用的吡唑和吡唑并[1,2-a]哒嗪。通过酸催化的超声辅助反应可以访问两个系列。这项研究中活性最高的化合物是两个新分子11和16,分别显示出有希望的选择性和16.50和20.1nM的适度IC50。这些化合物也停靠在COX-2酶的晶体结构(PDB ID:3LN1)中,以了解其结合方式。最后,使用DFT方法计算了化合物11和塞来昔布的Mulliken电荷和静电表面电势,以深入了解该化合物活性的分子决定因素。这些结果可能导致开发具有改进的选择性的新型COX-2抑制剂。
表征谱图
-
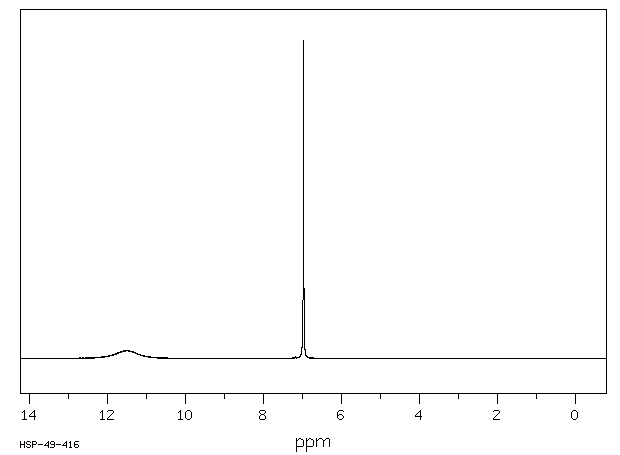
氢谱1HNMR
-
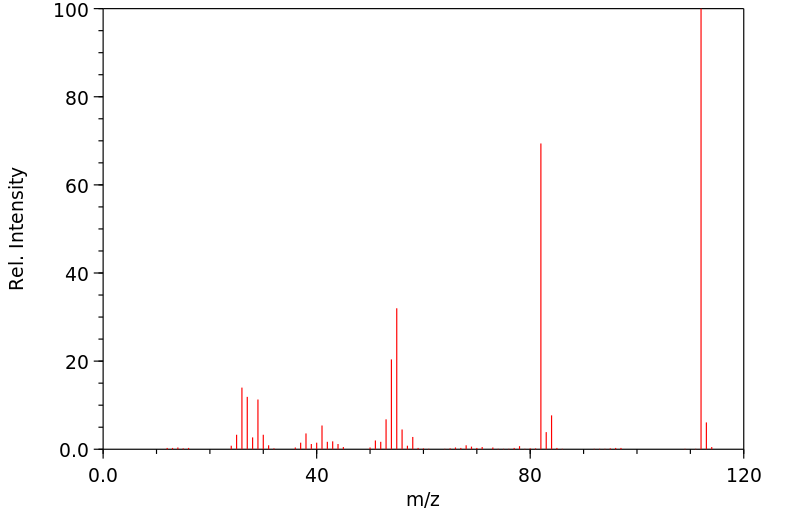
质谱MS
-
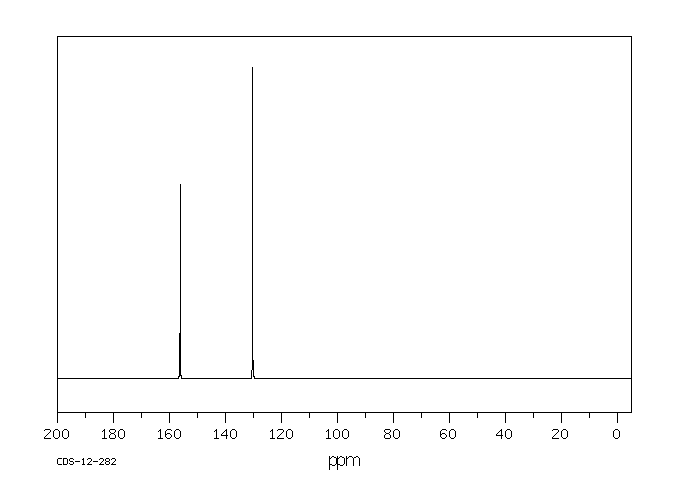
碳谱13CNMR
-
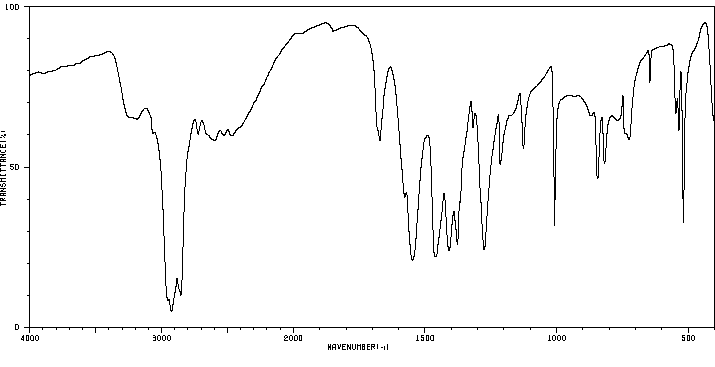
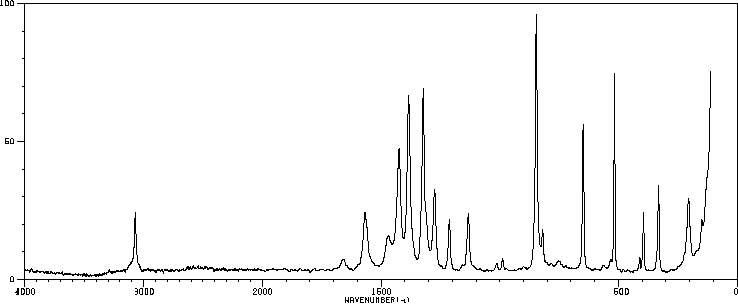
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息