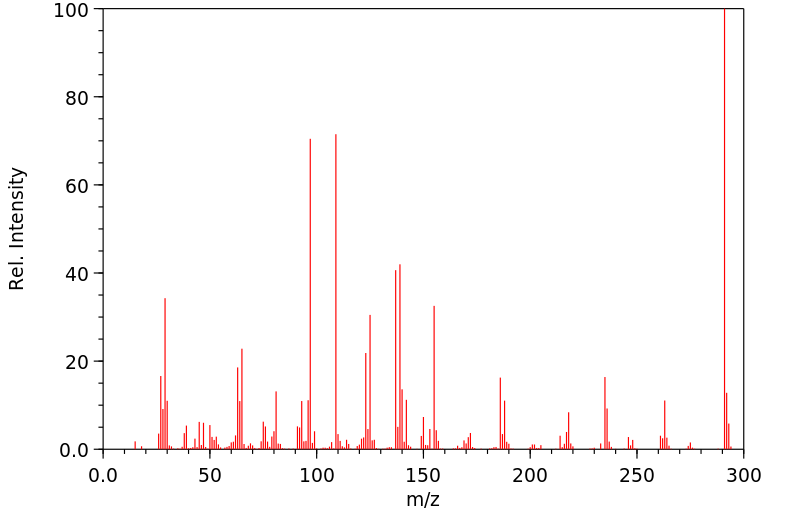
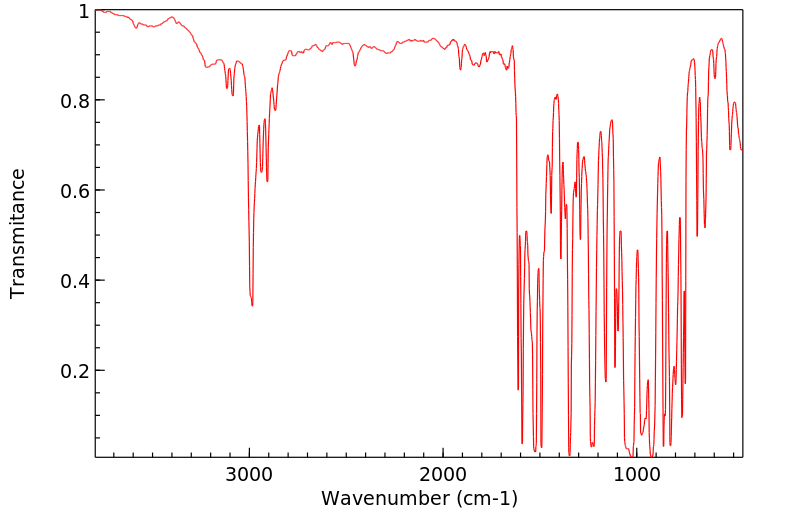
代谢
... 对硫磷的新陈代谢在多种生物体中得到了广泛的研究,包括微生物、植物、昆虫和哺乳动物。其生物转化/依赖于氧化激活,通过将硫代硫原子替换为氧原子以产生毒性。与这种中毒反应竞争的是水解反应,导致解毒。环上的硝基团也可以被还原,特别是在牛瘤胃液中,还原为氨基团;这导致氨基对硫磷的产生... 氧化激活主要发生在肝脏。许多研究已经表明,在肝脏微粒体混合功能氧化酶系统中,还原型烟酰胺腺嘌呤二核苷酸磷酸和氧气的存在是负责这一反应的。已经证明,对硫磷转化为对氧磷可以在体内和体外发生。导致对硫磷解毒的降解反应涉及脱芳基化。产生的二甲磷酰基酸基本上是无毒的。
THE METABOLISM OF ... PARATHION ... HAS BEEN EXTENSIVELY STUDIED IN A WIDE VARIETY OF ORGANISMS, INCL MICROORGANISMS, PLANTS, INSECTS & MAMMALS. ... /ITS BIOTRANSFORMATION/ DEPENDS ON OXIDATIVE ACTIVATION BY REPLACEMENT OF THIONO SULFUR WITH OXYGEN FOR ... TOXICITY. COMPETING WITH THIS INTOXICATION REACTION ARE HYDROLYSIS REACTIONS THAT RESULT IN DETOXIFICATION. THE RING NITRO GROUP CAN ALSO BE REDUCED, PARTICULARLY IN BOVINE RUMEN FLUID, TO AMINO GROUP; THIS RESULTS IN AMINO PARATHION ... OXIDATIVE ACTIVATION TAKES PLACE PRIMARILY IN LIVER. A NUMBER OF ... /STUDIES/ HAVE SHOWN THAT, IN LIVER MICROSOMAL MIXED FUNCTION OXIDASE SYSTEMS, PRESENCE OF REDUCED NICOTINAMIDE ADENINE DINUCLEOTIDE PHOSPHATE & OXYGEN ARE RESPONSIBE FOR THIS REACTION. THE CONVERSION OF PARATHION TO PARAOXON HAS BEEN DEMONSTRATED ... IN VIVO & IN VITRO. DEGRADATIVE REACTIONS THAT RESULT IN DETOXIFICATION OF PARATHION ... INVOLVE ... DEARYLATION. THE RESULTING ... DIMETHYL PHOSPHORIC ACIDS ARE ESSENTIALLY NONTOXIC.
来源:Hazardous Substances Data Bank (HSDB)