6-硝基苯[a]嵌二萘 | 63041-90-7
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:255.5°C
-
沸点:438.82°C (rough estimate)
-
密度:1.2310 (rough estimate)
-
颜色/状态:Orange-yellow needles, recrystallzed from benzene; yellow crystalline solid; orange crystals
-
溶解度:Limited solubility in toluene and benzene
-
蒸汽压力:1.0X10-10 mm Hg at 25 °C (est)
-
分解:When heated to decomposition it emits toxic fumes of NOx.
-
保留指数:490.9
计算性质
-
辛醇/水分配系数(LogP):6.6
-
重原子数:23
-
可旋转键数:0
-
环数:5.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:45.8
-
氢给体数:0
-
氢受体数:2
ADMET
安全信息
-
海关编码:2904209090
-
储存条件:| 2-8°C |
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 6-氨基苯并(a)芘 6-aminobenzopyrene 7428-83-3 C20H13N 267.33
反应信息
-
作为反应物:描述:参考文献:名称:Isocyanates of 3,4-Benzpyrene and 10-Methyl-1,2-benzanthracene摘要:DOI:10.1021/ja01847a062
-
作为产物:描述:6-nitro-7,8,9,10-tetrahydrobenzopyrene 在 2,3-二氯-5,6-二氰基-1,4-苯醌 作用下, 以 苯 为溶剂, 反应 15.0h, 以85%的产率得到6-硝基苯[a]嵌二萘参考文献:名称:1-,3-和6-硝基苯并[a] py的合成,光谱分析和诱变性。摘要:合成了诱变性环境污染物1-,3-和6-硝基苯并[a] re。在环境温度下用硝酸钠在三氟乙酸和乙酸酐中硝化7,8,9,10-四氢苯并[a] re,得到1-,3-和6-硝基-7,8,9,10-的混合物四氢苯并[a] py,通过色谱分离。用2,3-二氯-4,5-二氰基-1,6-苯醌对分离的硝基四氢苯并[a] py进行脱氢,可高产率地产生1-,3-和6-硝基苯并[a] py。将这些化合物的光谱数据与直接硝化苯并[a] py的光谱数据进行比较,证实1-和3-硝基苯并[a]]确实是后者反应的次要产物。该证实还证实1-和3-硝基苯并[a] py是在模型大气中形成的次要硝化的苯并[a] py的硝化产物。在鼠微粒体(S9)活化系统存在下,鼠伤寒沙门氏菌测试株TA98和TA100中的1-,3-和6-硝基苯并[a] re具有致突变性。在这些菌株中,1-硝基苯并[3-] py和6-硝基苯并[a] py均不是直接作DOI:10.1021/jm00375a012
文献信息
-
Chemical Oxidation of Nitrated Polycyclic Aromatic Hydrocarbons: Hydroxylation with Superoxide Anion Radical作者:Kiyoshi Fukuhara、Naoki MiyataDOI:10.1021/tx00043a003日期:1995.1Nitrated polycyclic aromatic hydrocarbon (nitroPAH) is a potent mutagen which is reductively and/or oxidatively metabolized. Biological oxidation of nitroPAH, such as hydroxylation and epoxidation, is known, but chemical oxidation has been reported in only a few papers. NitroPAH is barely oxidized by various chemical oxidants because of the electron deficient property of the aromatic ring with the nitro硝化的多环芳烃(nitroPAH)是一种强力的诱变剂,可以通过还原和/或氧化方式代谢。硝基PAH的生物氧化(例如羟基化和环氧化)是已知的,但是仅在几篇论文中报道了化学氧化。由于具有硝基取代基的芳环的电子不足特性,NitroPAH几乎不会被各种化学氧化剂氧化。超氧阴离子自由基的亲核反应性是已知的,因此本研究中进行了硝基PAH与化学生成的超氧阴离子自由基的氧化。当1-硝基py与KO2 / 18-crown-6在二甲基甲酰胺中反应时,可以制备产率得到5-,6-,8-和9-羟基-1-硝基py和1-羟基py。三个异构体二硝基nitro,3-硝基荧蒽,6-硝基苯并[a] py,和6-硝基铬被氧化为羟基衍生物,其中一些对应于硝基PAH的氧化代谢产物。用三氟过氧乙酸将二硝基吡啶氧化,得到K区氧化产物。
-
Synthesis, chemical properties and mutagenicity of 1,6- and 3,6-dinitrobenzo(a)pyrenes.作者:Kiyoshi FUKUHARA、Naoki MIYATA、Michiko MATSUI、Keiko MATSUI、Motoi Jr. ISHIDATE、Shozo KamiyaDOI:10.1248/cpb.38.3158日期:——Nitration of benzo[a]pyrene (BaP) with HNO3 (d = 1.38) produced a mixture of dinitroBaPs (1,6- and 3,6-isomers) and mononitroBaPs (1-, 3- and 6-isomers). Pure 1,6-dinitroBaP and 3,6-dinitroBaP were obtained by the reduction of the dinitroBaPs mixture with NaSH to yield the separable products 1-amino-6-nitroBaP and 3-amino-6-nitroBaP, followed by conversion to dinitroBaPs via the the diazonium salts苯并[a] py(BaP)与HNO3(d = 1.38)硝化产生了dinitroBaPs(1,6-和3,6-异构体)和mononitroBaPs(1-,3-和6-异构体)的混合物。通过用NaSH还原dinitroBaPs混合物,得到纯的1,6-dinitroBaP和3,6-dinitroBaP,得到可分离的产物1-amino-6-nitroBaP和3-amino-6-nitroBaP,然后通过转化为dinitroBaPs重氮盐。测量了与二硝基BaPs的单电子还原相对应的半波电势(E1 / 2),并讨论了这些值与诱变性之间的关系。
-
Atmospheric Heterogeneous Reactions of Benzo(a)pyrene作者:M. Cazaunau、K. Le Ménach、H. Budzinski、E. VillenaveDOI:10.1524/zpch.2010.6145日期:2010.8.1
Abstract This experimental study deals with heterogeneous reactions of benzo(a)pyrene (BaP) with ozone, nitrogen dioxide and hydroxyl radicals. BaP was adsorbed on silica particles chosen here as a model of mineral atmospheric particles. Compound extractions were assisted by focused microwave and analyses were performed by gas chromatography coupled with mass spectroscopy in single ion monitoring mode. Pseudo-first order rate constants were obtained from the fit of experimental decays of particulate-BaP concentration versus reaction time. Second order rate constants were determined considering the different oxidant gaseous concentrations except for the case of hydroxyl radicals where only a pseudo-first order rate constant was proposed. Values obtained at room temperature are (2.1±0.5)×10−15 cm3 molecule−1 s−1 for (BaP + ozone), (5.8±1.4)×10−16 cm3 molecule−1 s−1 for (BaP + nitrogen dioxide) and (3.4±0.8)×10−2 s−1 for (BaP + OH) reactions. Products have only been investigated for the NO2 and the OH (in the presence of NOx) reactions. 1-, 3- and 6-nitrobenzo(a)pyrenes were detected as degradation products and quantified. Reaction rate constants for product formation are (3.7±0.9)×10−16 cm3 molecule−1 s−1 for 6-NBaP, (2.2±0.6)×10−17 cm3 molecule−1 s−1 for 1-NBaP and (5.3±1.3)×10−17 cm3 molecule−1 s−1 for 3-NBaP. 1-, 3- and 6-nitroBaP account respectively for approximately 5%, 12% and 83% of total nitrated species. If in the presence of only nitrogen dioxide, BaP was totally degraded within few minutes, only 20 to 25 % of the initial BaP led to nitrated compounds when reacting with OH (in the presence of NOx).
摘要:本实验研究了苯并(a)芘(BaP)与臭氧、二氧化氮和羟基自由基的异质反应。BaP被吸附在二氧化硅颗粒上,作为大气矿物颗粒的模型。化合物的提取采用了聚焦微波辅助,分析采用气相色谱-质谱联用技术,在单离子监测模式下进行。通过实验浓度衰减曲线与反应时间的拟合,得到了伪一级速率常数。考虑不同氧化剂气态浓度,确定了二级速率常数,但对于羟基自由基的情况,只提出了伪一级速率常数。在室温下,得到的值为(BaP+臭氧)为(2.1±0.5)×10−15 cm3 molecule−1 s−1,(BaP+二氧化氮)为(5.8±1.4)×10−16 cm3 molecule−1 s−1,(BaP+OH)反应为(3.4±0.8)×10−2 s−1。仅对NO2和OH(在NOx存在下)反应的产物进行了研究。检测到并量化了1-、3-和6-硝基苯并(a)芘作为降解产物。产物形成的反应速率常数为6-NBaP为(3.7±0.9)×10−16 cm3 molecule−1 s−1,1-NBaP为(2.2±0.6)×10−17 cm3 molecule−1 s−1,3-NBaP为(5.3±1.3)×10−17 cm3 molecule−1 s−1。1-、3-和6-硝基苯并(a)芘分别占总硝化物的约5%、12%和83%。如果只有二氧化氮存在,BaP会在几分钟内完全降解,但在OH(在NOx存在下)反应时,只有20%至25%的初始BaP会生成硝化化合物。 -
Mutagenic nitrated benzo[a]pyrene derivatives in the reaction product of benzo[a]pyrene in NO2–air in the presence of O3 or under photoirradiation作者:Satoko Ishii、Yoshiharu Hisamatsu、Koji Inazu、Takaaki Kobayashi、Ken-ichi AikaDOI:10.1016/s0045-6535(00)00029-1日期:2000.12should be considered. Benzo[a]pyrene lactones were identified in a highly mutagenic fraction of the products of the dark reaction in the presence of O3 and photoreaction and a nitrobenzo[a]pyrene lactone was also identified in a highly mutagenic fraction of the dark reaction products in the presence of O3. Nitrated oxygenated benzo[a]pyrene derivatives such as nitrobenzo[a]pyrene lactone were considered为了阐明硝化产物在各种条件下在NO2-空气中苯并[a] py的反应产物的直接诱变活性的贡献,沉积在过滤器中的BaP的异相反应在含有10 ppm 的空气中具有在黑暗中或在光照射下进行。通过气相色谱分析了反应产物,并且在不存在S9混合物的情况下,通过制备型HPLC对产物的诱变性进行了鼠伤寒沙门氏菌TA98和YG1024菌株的诱变分析。3,6-二硝基苯并[a] py和1,3-二硝基苯并[a] py是很强的直接作用诱变剂,在很大程度上决定了深色反应产物在NO 2-空气中的总直接作用诱变性。另一方面,在O3存在下的暗反应和在 -空气中的光反应都导致形成的硝基苯并[a] py比在不存在O3的暗反应中观察到的要少得多。这些结果表明,在这些反应中,应考虑其他直接作用的诱变剂对产物的总直接作用诱变性的贡献。在存在O3和光反应的情况下,在黑暗反应产物的高度诱变部分中鉴定出苯并[a] py内酯,并且在
-
Packaging Inserts with Myoglobiin Blooming Agents, Packages and Methods of Packaging申请人:Curwood, Inc.公开号:EP1905584A2公开(公告)日:2008-04-02Food packaging inserts comprising a myoglobin blooming agent that promote or preserve the desirable appearance of food products, food packages, and methods of food packing comprising the same, are provided.本发明提供了由肌红蛋白绽放剂组成的食品包装插件,可促进或保持食品、食品包装的理想外观,以及由其组成的食品包装方法。
表征谱图
-
氢谱1HNMR
-
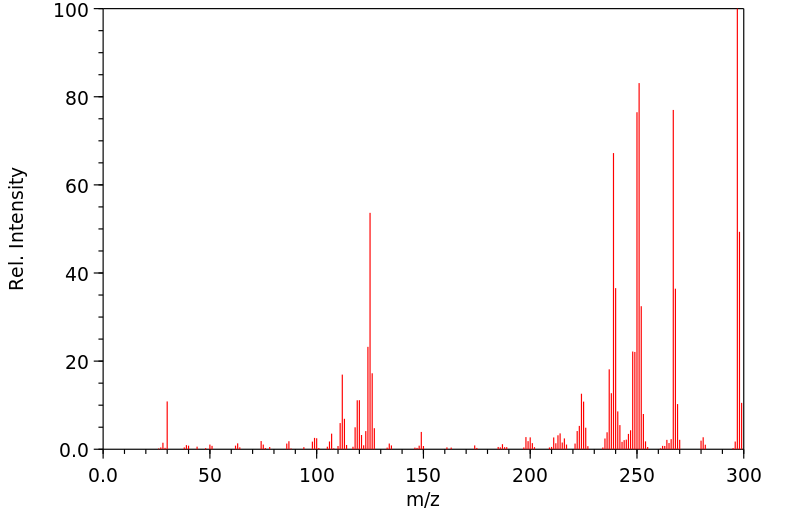
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息