对硝基苯硫醚 | 1223-31-0
中文名称
对硝基苯硫醚
中文别名
二(对硝基苯)硫醚;双(4-硝基苯基)硫醚;双(4-硝基苯)硫
英文名称
bis(4-nitrophenyl)sulfide
英文别名
4,4'-dinitrodiphenyl sulfide;bis(4-nitrophenyl)sulfane;di(4-nitrophenyl) sulfide;p-nitrophenyl sulfide;1-nitro-4-(4-nitrophenyl)sulfanylbenzene
CAS
1223-31-0
化学式
C12H8N2O4S
mdl
MFCD00039745
分子量
276.273
InChiKey
ZZTJMQPRKBNGNX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:160°C
-
沸点:487.4±30.0 °C(Predicted)
-
密度:1.4634 (rough estimate)
-
溶解度:溶于丙酮
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,没有已知危险反应。请避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:19
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:117
-
氢给体数:0
-
氢受体数:5
安全信息
-
危险类别码:R23/24/25
-
危险品运输编号:2811
-
RTECS号:WQ2150000
-
海关编码:2930909090
-
安全说明:S26,S36/37/39
-
储存条件:请将贮藏器密封保存,并存放在阴凉干燥处。同时,确保工作环境具备良好的通风或排气设施。
SDS
Bis(4-nitrophenyl) Sulfide Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Bis(4-nitrophenyl) Sulfide
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Acute toxicity (Oral) Category 4
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Harmful if swallowed
Precautionary statements:
[Prevention] Do not eat, drink or smoke when using this product.
Wash hands thoroughly after handling.
IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell.
[Response]
Rinse mouth.
Dispose of contents/container through a waste management company authorized by
[Disposal]
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Bis(4-nitrophenyl) Sulfide
Percent: >99.0%(GC)
CAS Number: 1223-31-0
Synonyms: 4,4'-Dinitrodiphenyl Sulfide , Di(4-nitrophenyl) Sulfide
Chemical Formula: C12H8N2O4S
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Bis(4-nitrophenyl) Sulfide
Section 4. FIRST AID MEASURES
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Call a POISON CENTER or doctor/physician if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
Colour: Pale yellow - Reddish yellow
No data available
Odour:
pH: No data available
Melting point/freezing point:160°C
Bis(4-nitrophenyl) Sulfide
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents]
Soluble: Acetone
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Sulfur oxides
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: orl-rat LD50:1490 mg/kg
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
RTECS Number: WQ2150000
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Bis(4-nitrophenyl) Sulfide
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Bis(4-nitrophenyl) Sulfide
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Acute toxicity (Oral) Category 4
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Harmful if swallowed
Precautionary statements:
[Prevention] Do not eat, drink or smoke when using this product.
Wash hands thoroughly after handling.
IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell.
[Response]
Rinse mouth.
Dispose of contents/container through a waste management company authorized by
[Disposal]
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Bis(4-nitrophenyl) Sulfide
Percent: >99.0%(GC)
CAS Number: 1223-31-0
Synonyms: 4,4'-Dinitrodiphenyl Sulfide , Di(4-nitrophenyl) Sulfide
Chemical Formula: C12H8N2O4S
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Bis(4-nitrophenyl) Sulfide
Section 4. FIRST AID MEASURES
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Call a POISON CENTER or doctor/physician if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
Colour: Pale yellow - Reddish yellow
No data available
Odour:
pH: No data available
Melting point/freezing point:160°C
Bis(4-nitrophenyl) Sulfide
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents]
Soluble: Acetone
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Sulfur oxides
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: orl-rat LD50:1490 mg/kg
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
RTECS Number: WQ2150000
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Bis(4-nitrophenyl) Sulfide
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 二-(4-硝基-苯基)亚砜 4,4'-dinitrodiphenyl sulfoxide 1774-38-5 C12H8N2O5S 292.272 4-硝基苯硫醇 para-nitrobenzenethiol 1849-36-1 C6H5NO2S 155.177 二(对硝基苯)硫砜 4-nitrophenyl sulfone 1156-50-9 C12H8N2O6S 308.271 4,4'-二硝基二苯二硫醚 di(p-nitrophenyl) disulfide 100-32-3 C12H8N2O4S2 308.339 —— 1-Nitro-4-[5-(4-nitrophenyl)sulfanylpentylsulfanyl]benzene 118542-94-2 C17H18N2O4S2 378.473 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-氨基-4'-硝基二苯硫醚 4-amino-4'-nitrodiphenyl sulfide 101-59-7 C12H10N2O2S 246.29 4-硝基苯基苯硫 4-nitrophenyl phenyl sulfide 952-97-6 C12H9NO2S 231.275 二-(4-硝基-苯基)亚砜 4,4'-dinitrodiphenyl sulfoxide 1774-38-5 C12H8N2O5S 292.272 —— 4-cyanophenyl (4'-nitrophenyl) sulfide 21969-10-8 C13H8N2O2S 256.285 —— 4-acetylamino-4'-nitrodiphenyl sulfide 7467-51-8 C14H12N2O3S 288.327 二(对硝基苯)硫砜 4-nitrophenyl sulfone 1156-50-9 C12H8N2O6S 308.271 4,4-二氨基二苯硫醚 4,4'-thiobisaniline 139-65-1 C12H12N2S 216.307 4-氨基二苯基硫化物 4-aminophenyl phenyl sulfide 1135-14-4 C12H11NS 201.292 4,4'-二硝基二苯二硫醚 di(p-nitrophenyl) disulfide 100-32-3 C12H8N2O4S2 308.339 4-[(4-硝基苯基)二硫烷基]苯胺 4-aminophenyl 4'-nitrophenyl disulfide 40897-48-1 C12H10N2O2S2 278.356 —— p-nitrobenzenesulphinamide 68597-89-7 C6H6N2O3S 186.191 - 1
- 2
反应信息
-
作为反应物:描述:对硝基苯硫醚 在 硼烷铵络合物 、 nickel(II) chloride hexahydrate 、 1,2-双(二苯基膦)乙烷 作用下, 以 乙醇 为溶剂, 以88 %的产率得到4,4-二氨基二苯硫醚参考文献:名称:均相镍催化氨-硼烷功能化硝基芳烃化学选择性转移氢化摘要:以NH 3 BH 3为氢源,成功建立了均相Ni催化硝基芳烃高选择性转移加氢反应。选择性还原多种官能团,以良好到高产率提供相应的苯胺。此外,可以制备否则难以获得的药物活性化合物。DOI:10.1039/d3cc05173k
-
作为产物:描述:4-硝基苯基乙酸酯 在 copper(l) iodide 、 sulfur 、 sodium t-butanolate 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 5.0h, 以90%的产率得到对硝基苯硫醚参考文献:名称:以S8为硫源时,通过C–O键的裂解,形成铜催化的C–S键的形成摘要:摘要 提出了一种有用且适用的通过C–O键活化一锅无味合成不对称和对称二芳基硫化物的方法。首先,报道了一种新的有效方法,用于合成不对称硫化物,该方法使用酚酯(如乙酸酯,甲苯磺酸酯和三氟甲磺酸酯)的交叉偶联反应,并以芳基硼酸或三苯基氯化锡为偶联伙伴。取决于反应,发现S 8 / KF或S 8 / NaO t -Bu体系在铜盐存在下和在聚乙二醇中作为绿色溶剂是硫的有效来源。然后,以S 8为硫源和NaO t由酚类化合物合成对称的二芳基硫醚描述了在N 2下在120°C的无水DMF中的-Bu 。通过这些方案,与不直接将硫醇和芳基卤化物用于制备硫化物的现有方案相比,各种不对称和对称硫化物的合成变得更加容易。 提出了一种有用且适用的通过C–O键活化一锅无味合成不对称和对称二芳基硫化物的方法。首先,报道了一种新的有效方法,用于合成不对称硫化物,该方法使用酚酯(如乙酸酯,甲苯磺酸酯和三氟甲磺酸酯)的交叉偶联反应DOI:10.1055/s-0036-1588508
文献信息
-
Fe3O4-AMPD-Pd: A novel and efficient magnetic nanocatalyst for synthesis of sulfides and oxidation reactions作者:Taiebeh Tamoradi、Nazanin Moeini、Mohammad Ghadermazi、Arash Ghorbani-ChoghamaraniDOI:10.1016/j.poly.2018.07.002日期:2018.10Abstract A novel magnetic nanoparticle was synthesized with effective catalytic properties and recyclable ability. This heterogeneous nanocatalyst was identified using Fourier transform infrared, scanning electron microscopies, X‐ray diffraction, vibrating sample magnetometer, inductively coupled plasma atomic emission spectroscopy and thermogravimetric analysis methods. The nanocatalyst was used for
-
Copper based on diaminonaphthalene-coated magnetic nanoparticles as robust catalysts for catalytic oxidation reactions and C–S cross-coupling reactions作者:Nasrin Yarmohammadi、Mohammad Ghadermazi、Roya MozafariDOI:10.1039/d1ra01029h日期:——copper(II) on the surface of 1,8-diaminonaphthalene (DAN)-coated magnetic nanoparticles provides a highly active catalyst for the oxidation reaction of sulfides to sulfoxides and the oxidative coupling of thiols to disulfides using hydrogen peroxide (H2O2). This catalyst was also applied for the one-pot synthesis of symmetrical sulfides via the reaction of aryl halides with thiourea as the sulfur source在这项工作中,将铜( II )固定在1,8-二氨基萘(DAN)包覆的磁性纳米粒子表面上,为硫化物氧化成亚砜以及使用氢气将硫醇氧化偶联成二硫化物提供了高活性催化剂。过氧化物(H 2 O 2 )。该催化剂还可用于芳基卤化物与硫脲作为硫源在NaOH存在下反应一锅合成对称硫化物,取代了以往的强碱性和苛刻的反应条件。在最佳条件下,亚砜、对称硫化物和二硫化物的合成收率分别约为99%、95%和96%,且选择性最高。本发明的非均相铜基催化剂具有催化剂易于回收、产物易于分离、催化剂分离过程中产物浪费少等优点。这种多相纳米催化剂通过 FESEM、FT-IR、VSM、XRD、EDX、ICP 和 TGA 进行了表征。此外,回收的催化剂可以重复使用多次,并且经济有效。
-
[EN] METHODS FOR PRODUCING ARYLSULFUR PENTAFLUORIDES<br/>[FR] PROCÉDÉS DE PRODUCTION DE PENTAFLUORURES DE SOUFRE ARYLÉS申请人:IM & T RES INC公开号:WO2010014665A1公开(公告)日:2010-02-04Novel methods for preparing arylsulfur pentafluorides are disclosed. Arylsulfur halotetrafluoride is reacted with a fluoride source under hydrous conditions to form an arylsulfur pentafluoride. The purification method is also disclosed.
-
A Highly Efficient Method for the Copper-Catalyzed Selective Synthesis of Diaryl Chalcogenides from Easily Available Chalcogen Sources作者:Yaming Li、Caiping Nie、Huifeng Wang、Xiaoying Li、Francis Verpoort、Chunying DuanDOI:10.1002/ejoc.201101121日期:2011.12copper-catalyzed C–S or C–Se bond formation between aryl iodides and easily available chalcogen sources leading to diaryl chalcogenides is reported. A variety of symmetrical diaryl sulfides and diaryl selenides were synthesized in good to excellent yields. Unsymmetrical diaryl sulfides were also obtained in moderate yields from two different aryl iodides by a one-pot tandem process. This strategy was
-
Use of Base Control To Provide High Selectivity between Diaryl Thioether and Diaryl Disulfide for C–S Coupling Reactions of Aryl Halides and Sulfur and a Mechanistic Study作者:Hsing-Ying Chen、Wei-Te Peng、Ying-Hsien Lee、Yu-Lun Chang、Yen-Jen Chen、Yi-Chun Lai、Nai-Yuan Jheng、Hsuan-Ying ChenDOI:10.1021/om400784w日期:2013.10.14Previous studies have reported that S-arylation produces diaryl disulfide when the precursors include sulfur powder and aryl halide using CuI as the catalyst. However, our research has revealed that the use of different bases in the above S-arylation process results in the coproduction of diarylsulfane and diaryldisulfane. In addition, we have demonstrated that the ratio of the two products can be先前的研究报道,当前体包括硫粉和使用CuI作为催化剂的芳基卤化物时,S-芳基化反应将生成二芳基二硫化物。但是,我们的研究表明,在上述S-芳基化过程中使用不同的碱会导致联产二芳基硫烷和二芳基二硫烷。另外,我们证明了可以通过选择碱的碱度来控制两种产物的比例。1个1 H NMR谱表明,二芳基二硫醚是第一产物,通过CuI催化与芳基卤化物反应生成二芳基硫烷成为试剂。使用各种不同的碱,对各种芳基卤化物进行了测试,以提高二芳基硫烷和二芳基二硫烷之间的选择性,从而得出以下原理。弱碱,例如金属碳酸盐或乙酸盐,只会产生二芳基二硫醚;强碱(例如金属氢氧化物)会同时生成二芳基二硫烷和二芳基硫烷。根据DFT计算,氢氧根离子被碘离子交换并与铜键合,从而更强烈地影响铜电子以还原二芳基二硫化物。
表征谱图
-
氢谱1HNMR
-
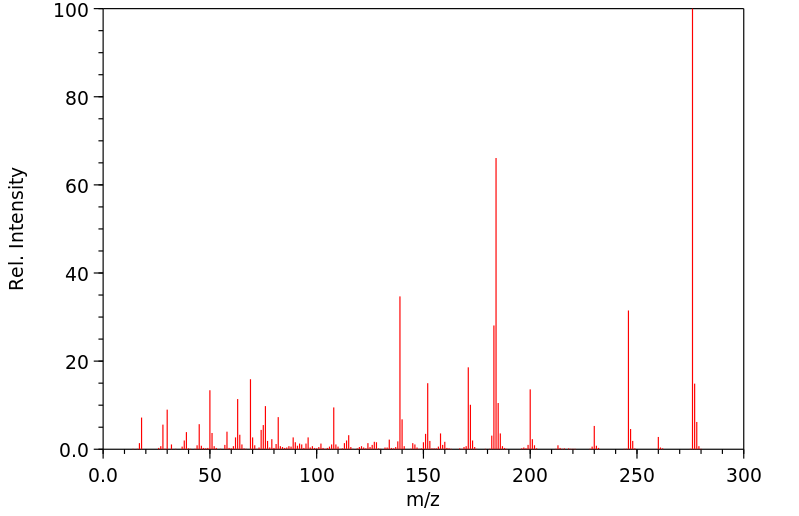
质谱MS
-
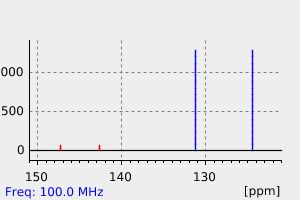
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Rp)-2-(叔丁硫基)-1-(二苯基膦基)二茂铁
(1E)-1-{4-[(4-氨基苯基)硫烷基]苯基}乙酮肟
颜料红88
颜料紫36
顺式-1,2-二(乙硫基)-1-丙烯
非班太尔-D6
雷西那得中间体
阿西替尼杂质J
阿西替尼杂质C
阿西替尼杂质4
阿西替尼杂质
阿西替尼
阿拉氟韦
阿扎毒素
阿嗪米特
阔草特
银(I)(6-氨基-2-(甲硫基)-5-亚硝基嘧啶-4-基)酰胺水合物
钾三氟[3-(苯基硫基)丙基]硼酸酯(1-)
邻甲苯基(对甲苯基)硫化物
避虫醇
连翘脂苷B
还原红 41
还原紫3
还原桃红R
达索尼兴
辛硫醚
辛-1,7-二炔-1-基(苯基)硫烷
西嗪草酮
萘,2-[(2,3-二甲基苯基)硫代]-
莫他哌那非
茴香硫醚
苯醌B
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-甲基苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2,6-二氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,2-[(2-硝基苯基)硫代]-
苯酚,3-氯-4-[(4-硝基苯基)硫代]-
苯酚,3-(乙硫基)-
苯酚,3,5-二[(苯基硫代)甲基]-
苯胺,4-[5-溴-3-[4-(甲硫基)苯基]-2-噻嗯基]-
苯胺,3-氯-4-[(1-甲基-1H-咪唑-2-基)硫代]-
苯胺,2-[(2-吡啶基甲基)硫代]-
苯硫醚-D10
苯硫胍
苯硫基乙酸
苯硫代磺酸S-(三氯乙烯基)酯
苯甲醇,2,3,4,5,6-五氟-a-[(苯基硫代)甲基]-,(R)-
苯甲酸,3-[[2-[(二甲氨基)甲基]苯基]硫代]-,盐酸
苯甲胺,5-氟-2-((3-甲氧苯基)硫代)-N,N-二甲基-,盐酸
苯甲二硫酸,4-溴苯基酯