4-硝基苯基苯硫 | 952-97-6
中文名称
4-硝基苯基苯硫
中文别名
4-硝基二苯硫醚;4-硝基苯基苯基硫化物
英文名称
4-nitrophenyl phenyl sulfide
英文别名
(4-nitrophenyl)(phenyl)sulfane;4-nitrodiphenyl sulfide;p-Nitrophenyl phenyl sulfide;1-nitro-4-(phenylsulfanyl)benzene;4-nitro-1-(phenylthio)benzene;1-nitro-4-(phenylthio)benzene;1-nitro-4-phenylsulfanylbenzene
CAS
952-97-6
化学式
C12H9NO2S
mdl
MFCD00024700
分子量
231.275
InChiKey
RJCBYBQJVXVVKB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:52-55 °C(lit.)
-
沸点:288.2 °C(Press: 100 Torr)
-
密度:1.2840 (rough estimate)
-
稳定性/保质期:
在常温常压下保持稳定,应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):4.4
-
重原子数:16
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:71.1
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2930909090
-
RTECS号:WQ5620000
-
危险性防范说明:P261,P301+P312,P302+P352,P304+P340,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:请将容器密封保存,并存放在阴凉、干燥处。
SDS
| Name: | p-Nitrophenyl phenyl sulfide 98% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 952-97-6 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 952-97-6 | 4-Nitrophenyl phenyl sulfide | 98 | 213-462-5 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
Dust may cause mechanical irritation. The toxicological properties of this material have not been fully investigated.
Skin:
May cause skin irritation. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause digestive tract disturbances. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 952-97-6: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystalline powder
Color: yellow to green
Odor: None reported.
pH: Not available.
Vapor Pressure: 2 mmHg @ 182 C
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 56.00 - 58.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C12H9NO2S
Molecular Weight: 231.27
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, oxides of sulfur, carbon dioxide, nitrogen, hydrogen sulfide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 952-97-6: WQ5620000 LD50/LC50:
Not available.
Carcinogenicity:
4-Nitrophenyl phenyl sulfide - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 952-97-6: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 952-97-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 952-97-6 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-氨基-4'-硝基二苯硫醚 4-amino-4'-nitrodiphenyl sulfide 101-59-7 C12H10N2O2S 246.29 对硝基苯硫醚 bis(4-nitrophenyl)sulfide 1223-31-0 C12H8N2O4S 276.273 —— S-p-nitrophenyl-S-phenylsulfilimine 36744-95-3 C12H10N2O2S 246.29 —— 1-(benzenesulfinyl)-4-nitrobenzene 955-45-3 C12H9NO3S 247.274 4-硝基苯硫醇 para-nitrobenzenethiol 1849-36-1 C6H5NO2S 155.177 4-硝基联苯砜 4-nitrophenyl phenyl sulfone 1146-39-0 C12H9NO4S 263.274 4-硝基苯亚磺酰氯 4-nitrobenzenesulfenyl chloride 937-32-6 C6H4ClNO2S 189.622 —— 2-(4-nitrobenzenesulfenyl)pyrrole 150810-52-9 C10H8N2O2S 220.252 4-氯-4’-硝基二苯醚 1-chloro-4-((4-nitrophenyl)sulfonyl)benzene 39055-84-0 C12H8ClNO4S 297.719 4,4'-二硝基二苯二硫醚 di(p-nitrophenyl) disulfide 100-32-3 C12H8N2O4S2 308.339 —— fluoro(4-nitrophenyl)(phenyl)-λ6-sulfanenitrile 143885-01-2 C12H9FN2O2S 264.28 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— S-p-nitrophenyl-S-phenylsulfilimine 36744-95-3 C12H10N2O2S 246.29 —— 1-(benzenesulfinyl)-4-nitrobenzene 955-45-3 C12H9NO3S 247.274 —— 4-phenylthiophenylhydroxylamine 21229-99-2 C12H11NOS 217.291 —— (4-chloromethyl-phenyl)-(4-nitro-phenyl)-sulfide 100398-42-3 C13H10ClNO2S 279.747 4-硝基联苯砜 4-nitrophenyl phenyl sulfone 1146-39-0 C12H9NO4S 263.274 —— 4-acetylphenyl p-nitrophenyl sulfide 107558-16-7 C14H11NO3S 273.312 4-氨基二苯基硫化物 4-aminophenyl phenyl sulfide 1135-14-4 C12H11NS 201.292 —— 4-(4-nitro-phenylsulfanyl)-benzophenone 6317-77-7 C19H13NO3S 335.383 —— S-p-nitrophenyl-S-phenylsulfoximide 66429-81-0 C12H10N2O3S 262.289 —— (4-nitrophenyl)phenyl-N-(1,2,4-triazol-4-yl)sulfilimine 1370551-67-9 C14H11N5O2S 313.34 —— 4,4'-Bis-(phenylthio)-azoxybenzol 5333-73-3 C24H18N2OS2 414.552 —— N,N-dimethyl-4-(phenylthio)aniline 42881-80-1 C14H15NS 229.346 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:含有半醌和邻氨基苯酚苯并恶唑配体的双自由基氧代钼络合物摘要:我们报道了一种新的单核钼( IV )配合物MoOL BIS L SQ ,其中L SQ (2,4-二叔丁基邻半苯醌配体)是通过三齿的邻亚氨基半苯醌形式的反应制备的非无害苯并恶唑配体、 L BIS和MoO 2 (acac) 2 。通过 X 射线晶体学、元素分析、红外和紫外-可见光谱以及磁化率测量对该复合物进行了表征。 MoOL BIS L SQ的晶体结构显示出围绕金属中心的扭曲八面体几何形状,周围环绕着L BIS的一个 O 原子和两个 N 原子以及L SQ的两个 O 原子。 MoOL BIS L SQ的有效磁矩( μ eff )在290至2 K的温度范围内从2.36降至0.2 μB ,表明金属与配体中心不成对电子之间的反铁磁耦合引起的单重态基态。此外,后者导致了该复合体的 EPR 沉默。循环伏安法(CV)研究表明配体和金属中心的氧化还原过程。 MoOL BIS L SQ用作环己烯氧化裂解为己二DOI:10.1039/d0ra06351g
-
作为产物:描述:参考文献:名称:吡咯的硫基保护基和2-(2,4-二硝基苯)亚磺酰基和磺酰基吡咯的轻松脱保护摘要:简单的亚磺酰基和磺酰基作为吡咯的吸电子保护基的有效性已使用13 C NMR光谱法进行了分析,并通过考虑X射线晶体结构得到了证实。另外,已表明2,4-二硝基苯亚磺酰基和磺酰基是吡咯的有效的吸电子保护基,它们可以通过在温和和特定条件下用苯硫醇或硫醇盐处理而除去。DOI:10.1021/jo050077b
-
作为试剂:参考文献:名称:AROMATIC SULFIDE COMPOUNDS AND METHODS AND USE THEREOF摘要:本文描述了芳香族硫化物组合物及其使用方法,用于治疗或预防癌症。公开号:US20150266836A1
文献信息
-
Efficient copper(I)-catalyzed C–S cross-coupling of thiols with aryl halides in an aqueous two-phase system作者:Xin-Yan Zhang、Xiao-Yan Zhang、Sheng-Rong GuoDOI:10.1080/17415993.2010.547197日期:2011.2.1A mild and convenient C–S bond formation reaction catalyzed by CuI/L-proline in an aqueous two-phase system was achieved, providing a simple method for the synthesis of aryl sulfides in good yields.
-
Microwave-Assisted Simple and Efficient Ligand Free Copper Nanoparticle Catalyzed Aryl-Sulfur Bond Formation作者:Brindaban C. Ranu、Amit Saha、Ranjan JanaDOI:10.1002/adsc.200700289日期:2007.12.10A new protocol for the coupling of aryl iodides with thiophenols and alkanethiols catalyzed by copper nanoparticles under ligand-free condition has been developed. A variety of functionalized aryl sulfides are prepared in excellent yields under microwave irradiation for 5–7 min. A plausible radical mechanism has been suggested.
-
8-Hydroxyquinolin-N-oxide-Promoted Copper-Catalyzed C–S Cross-Coupling of Thiols with Aryl Iodides作者:Dayong Zhang、Sheng Jiang、Kun Su、Yatao Qiu、Yiwu YaoDOI:10.1055/s-0032-1317518日期:——8-Hydroxyquinolin- N -oxide was identified as a superior ligand for CuI-catalyzed C–S coupling reactions of aryl iodides with thiols to afford the corresponding thioethers in excellent yield. The method shows excellent chemoselectivity and high functional-group tolerance in both coupling partners.
-
Ferromagnetic nanoparticle-supported copper complex: A highly efficient and reusable catalyst for three-component syntheses of 1,4-disubstituted 1,2,3-triazoles and C-S coupling of aryl halides作者:Mohammad Mehdi Khodaei、Kiumars Bahrami、Farhat Sadat MeibodiDOI:10.1002/aoc.3714日期:2017.10A new nanocatalyst was synthesized by immobilization of 4′‐(4‐hydroxyphenyl)‐2,2′:6′,2″‐terpyridine/CuI complex on ferromagnetic nanoparticles through a surface modification (FMNPs@SiO2‐TPy‐Cu). This heterogeneous catalyst was characterized using various techniques including Fourier transform infrared and energy‐dispersive X‐ray spectroscopies, transmission and scanning electron microscopies, X‐ray
-
Copper(II)-faciliated synthesis of substituted thioethers and 5-substituted 1H-tetrazoles: Experimental and theoretical studies作者:Samaresh Layek、Bhumika Agrahari、Shuvankar Dey、Rakesh Ganguly、Devendra D. PathakDOI:10.1016/j.jorganchem.2019.06.008日期:2019.9thermo-gravimetric (TG) analysis and Cyclic Voltammetry. The molecular structures of both complexes have also been determined by single crystal X-ray crystallography, which confirmed the coordination of Schiff base ligands through N, O donor atoms and distorted square planar geometry around the Cu(II) ion. Both complexes were found to be good homogeneous catalysts for the synthesis of a wide range of substituted通过Cu(CH 3 COO)2的反应合成了基于苯甲酰肼的席夫碱连接的两种新的铜(II)配合物[Cu(L 1)2 ] (1)和[Cu(L 2)2 ] (2)。 ·具有各自的席夫碱配体1-[(4-硝基苯基)亚乙基]苯并酰肼(HL 1)或1-[(4-甲氧基苯基)亚乙基]苯并酰肼(HL 2)的H 2 O)。两种配合物均被分离为绿色固体,并通过元素分析,FT-IR,EPR,热重(TG)分析和循环伏安法进行了全面表征。两种配合物的分子结构也已通过单晶X射线晶体学测定,这证实了席夫碱配体通过N,O供体原子的配位和Cu(II)离子周围扭曲的正方形平面几何结构。发现这两种配合物都是合成大量取代硫醚和5-取代1 H的良好均相催化剂-四唑在低催化剂负载量(0.5摩尔%)下的收率分别为92%和93%。从DFT计算中可以看出,结合角和距离与实验结果相吻合。根据DFT研究计算得出,HOMO和LUMO之间的能量差分别为复合物1和复合物2的5
表征谱图
-
氢谱1HNMR
-
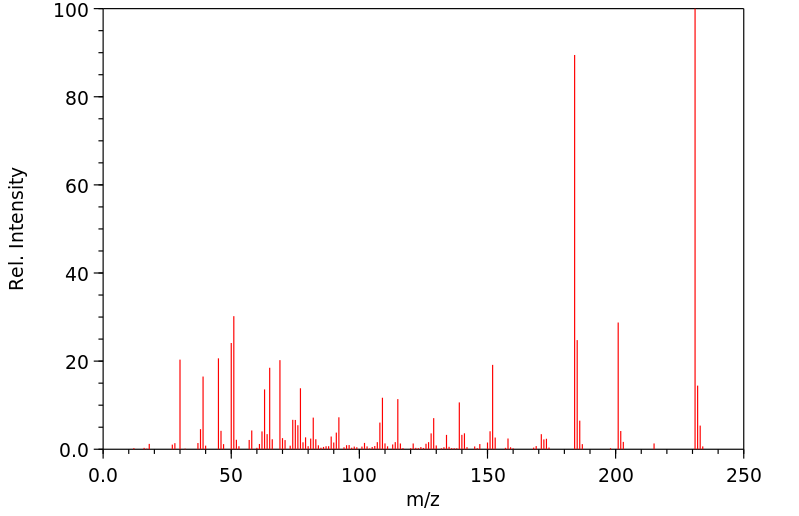
质谱MS
-
碳谱13CNMR
-
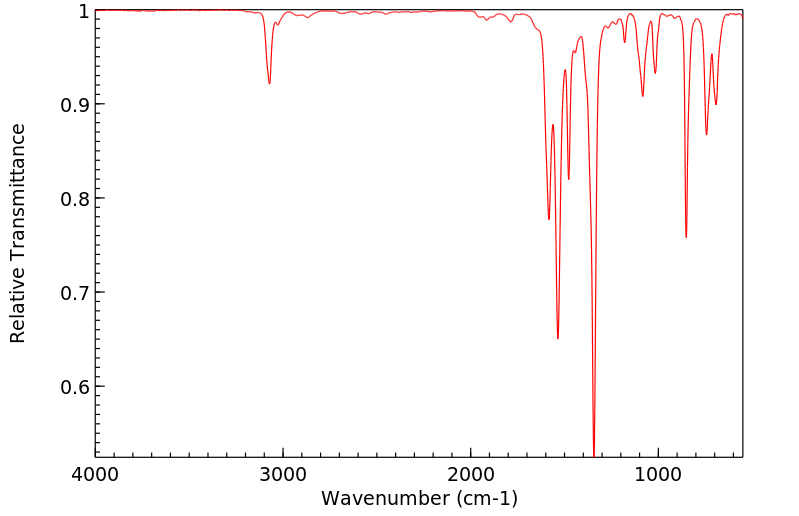
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Rp)-2-(叔丁硫基)-1-(二苯基膦基)二茂铁
(1E)-1-{4-[(4-氨基苯基)硫烷基]苯基}乙酮肟
颜料红88
颜料紫36
顺式-1,2-二(乙硫基)-1-丙烯
非班太尔-D6
雷西那得中间体
阿西替尼杂质J
阿西替尼杂质C
阿西替尼杂质4
阿西替尼杂质
阿西替尼
阿拉氟韦
阿扎毒素
阿嗪米特
阔草特
银(I)(6-氨基-2-(甲硫基)-5-亚硝基嘧啶-4-基)酰胺水合物
钾三氟[3-(苯基硫基)丙基]硼酸酯(1-)
邻甲苯基(对甲苯基)硫化物
避虫醇
连翘脂苷B
还原红 41
还原紫3
还原桃红R
达索尼兴
辛硫醚
辛-1,7-二炔-1-基(苯基)硫烷
西嗪草酮
萘,2-[(2,3-二甲基苯基)硫代]-
莫他哌那非
茴香硫醚
苯醌B
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-甲基苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2,6-二氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,2-[(2-硝基苯基)硫代]-
苯酚,3-氯-4-[(4-硝基苯基)硫代]-
苯酚,3-(乙硫基)-
苯酚,3,5-二[(苯基硫代)甲基]-
苯胺,4-[5-溴-3-[4-(甲硫基)苯基]-2-噻嗯基]-
苯胺,3-氯-4-[(1-甲基-1H-咪唑-2-基)硫代]-
苯胺,2-[(2-吡啶基甲基)硫代]-
苯硫醚-D10
苯硫胍
苯硫基乙酸
苯硫代磺酸S-(三氯乙烯基)酯
苯甲醇,2,3,4,5,6-五氟-a-[(苯基硫代)甲基]-,(R)-
苯甲酸,3-[[2-[(二甲氨基)甲基]苯基]硫代]-,盐酸
苯甲胺,5-氟-2-((3-甲氧苯基)硫代)-N,N-二甲基-,盐酸
苯甲二硫酸,4-溴苯基酯