四苯基硅烷 | 1048-08-4
中文名称
四苯基硅烷
中文别名
四苯矽[烷]
英文名称
tetraphenylsilane
英文别名
TPS
CAS
1048-08-4
化学式
C24H20Si
mdl
MFCD00014069
分子量
336.508
InChiKey
JLAVCPKULITDHO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:236 °C
-
沸点:228 °C
-
密度:1.078
-
闪点:193°C
-
稳定性/保质期:
在常温常压下保持稳定,应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):6.45
-
重原子数:25
-
可旋转键数:4
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
TSCA:Yes
-
安全说明:S22,S24/25
-
海关编码:29319090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:请将容器密封保存,并存放在阴凉、干燥处。
SDS
Tetraphenylsilane Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Tetraphenylsilane
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Tetraphenylsilane
Percent: >97.0%(GC)
CAS Number: 1048-08-4
Chemical Formula: C24H20Si
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Tetraphenylsilane
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Protect from moisture.
Store away from incompatible materials such as oxidizing agents.
Moisture-sensitive
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
Colour: White - Almost white
No data available
Odour:
pH: No data available
Melting point/freezing point:238°C
Boiling point/range: 166°C/0.01kPa
No data available
Flash point:
Flammability or explosive
limits:
Lower: No data available
No data available
Upper:
Relative density: No data available
Solubility(ies):
[Water] No data available
Tetraphenylsilane
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Silicon oxides
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
Tetraphenylsilane
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Tetraphenylsilane
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Tetraphenylsilane
Percent: >97.0%(GC)
CAS Number: 1048-08-4
Chemical Formula: C24H20Si
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Tetraphenylsilane
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Protect from moisture.
Store away from incompatible materials such as oxidizing agents.
Moisture-sensitive
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
Colour: White - Almost white
No data available
Odour:
pH: No data available
Melting point/freezing point:238°C
Boiling point/range: 166°C/0.01kPa
No data available
Flash point:
Flammability or explosive
limits:
Lower: No data available
No data available
Upper:
Relative density: No data available
Solubility(ies):
[Water] No data available
Tetraphenylsilane
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Silicon oxides
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
Tetraphenylsilane
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 三苯基硅烷 HSiPh3 789-25-3 C18H16Si 260.411 二苯基硅烷 diphenylsilane 775-12-2 C12H12Si 184.313 甲基三苯基硅烷 methyltriphenylsilane 791-29-7 C19H18Si 274.437 三苯基硅醇 triphenylhydroxysilane 791-31-1 C18H16OSi 276.41 —— triphenylsilyl bromide 6990-64-3 C18H15BrSi 339.307 三苯基氯硅烷 Triphenylchlorosilane 76-86-8 C18H15ClSi 294.856 三苯甲基氟硅烷 triphenylsilyl fluoride 379-50-0 C18H15FSi 278.401 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— Si(ph-3-NH2)4 18751-12-7 C24H24N4Si 396.567 4,4’-双(三苯基硅基)-1,1’-联苯 4,4'-bis(triphenylsilyl)biphenyl 18826-13-6 C48H38Si2 671.001 甲基三苯基硅烷 methyltriphenylsilane 791-29-7 C19H18Si 274.437 三苯基硅醇 triphenylhydroxysilane 791-31-1 C18H16OSi 276.41 —— triphenylsilyl bromide 6990-64-3 C18H15BrSi 339.307 三苯基氯硅烷 Triphenylchlorosilane 76-86-8 C18H15ClSi 294.856
反应信息
-
作为反应物:描述:参考文献:名称:Soschestwenskaja, Zhurnal Obshchei Khimii, 1956, vol. 26, p. 231; engl.Augs.S.247摘要:DOI:
-
作为产物:参考文献:名称:Gmelin Handbuch der Anorganischen Chemie, Gmelin Handbook: Si: MVol.C, 23, page 72 - 75摘要:DOI:
-
作为试剂:参考文献:名称:一种铁催化氧化烯合成酮的方法摘要:本发明公开了一种铁催化氧化烯合成酮的方法,属于催化合成技术和精细化学品合成领域。本发明的具体方法是在氢硅烷的促进作用下,以空气或氧气为氧化剂,铁催化氧化烯合成酮类化合物。本发明方法具有催化剂来源广泛、廉价和环保的优势;氧化剂来源广泛、廉价和不产生废物;反应条件温和、选择性高和产率高;底物来源广泛且稳定;底物官能团相容性好且底物的适用范围广;复杂烯分子能很好的转化成酮。在优化的反应条件之下,目标产品分离收率高达98%。公开号:CN106748690B
文献信息
-
Fluoride-Promoted Cross-Coupling of Chloro(mono-, di-, or triphenyl)germanes with Aryl Halides in “Moist” Toluene. Multiple Transfer of the Phenyl Group from Organogermane Substrates and Comparison of the Coupling Efficiencies of Chloro(phenyl)germanes with their Corresponding Stannane and Silane Counterparts作者:Jean-Philippe Pitteloud、Zun-Ting Zhang、Yong Liang、Laura Cabrera、Stanislaw F. WnukDOI:10.1021/jo101848f日期:2010.12.3efficient activation by fluoride to promote transfer of one, two, or three phenyl groups from the organogermanes. The corresponding chlorophenylstannanes were found to be more reactive than chlorophenylsilanes, which in turn were more effective than chlorophenylgermanes. One chloride ligand on the Ge or Si center allows efficient activation by fluoride to promote transfer of up to three aryl groups
-
Carbonylative coupling reaction of organofluorosilanes with organic halides promoted by fluoride ion and palldium catalyst作者:Yasuo Hatanaka、Satoshi Fukushima、Tamejiro HiyamaDOI:10.1016/s0040-4020(01)88878-5日期:1992.1Palladium-catalyzed carbonylative cross coupling reaction of organofluorosilanes with organic halides was achieved in the presence of fluoride ion and an atmospheric pressure of carbon monoxide. Alkenyl- or arylfluorosilanes effectively underwent this reaction with alkenyl or aryl iodides in moderate to good yields. Thus, highly functionalized ketones are readily available without protection of reactive
-
Reactivity of Hypervalent Species: Reactions of Anionic Penta-Coordinated Silicon Complexes towards Nucleophiles作者:Alain Boudin、Geneviève Cerveau、Claude Chuit、Robert J. P. Corriu、Catherine ReyeDOI:10.1246/bcsj.61.101日期:1988.1The reactivity of anionic penta-coordinated silicon complexes [RSi(O2C6H4-o)2]−Na+ 1 with nucleophilic reagents has been studied. 1 can be reduced to organosilanes RSiH3 by metallic hydrides. Reactions with an excess of Grignard or organolithium reagents (R′MgX or R′Li) gave tetraorganosilanes RSiR′3. When only two molar equivalents of Grignard reagents (R′MgX) or lithium reagents (R′Li) are added
-
Disproportionation reactions of organohydrosilanes in the presence of base catalysts作者:Masayoshi Itoh、Koji Inoue、Jun-ichi Ishikawa、Kenji IwataDOI:10.1016/s0022-328x(01)00755-0日期:2001.6Alkoxides, alkyl compounds, amides and hydrides of alkali metals (M) and barium, such as MOR, Ba(OR)2, n-BuM, PhM, MN(SiMe3)2 and MAlH4 showed high catalytic activities versus the disproportionation reactions of PhSiH3 to produce SiH4, Ph2SiH2 and Ph3SiH. A good correlation between the catalyst basicities and the catalytic activities was observed, and a reaction mechanism involving the metal hydride
-
Pentacoordinate silicon compounds. Reactions of silatranes with nucleophiles作者:G. Cerveau、C. Chuit、R.J.P. Corriu、N.K. Nayyar、C. ReyeDOI:10.1016/0022-328x(90)85408-q日期:1990.6studied. Substitution involving cleavage of equatorial SiO bonds is always observed. Silatranes exhibit reactivity quite different from that of analogous trialkoxysilanes or anionic pentacoordinate silicon compounds.
表征谱图
-
氢谱1HNMR
-
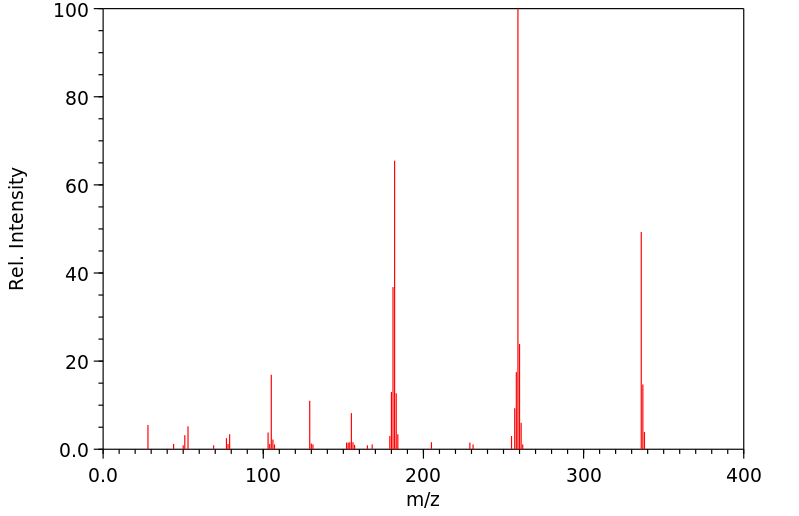
质谱MS
-
碳谱13CNMR
-
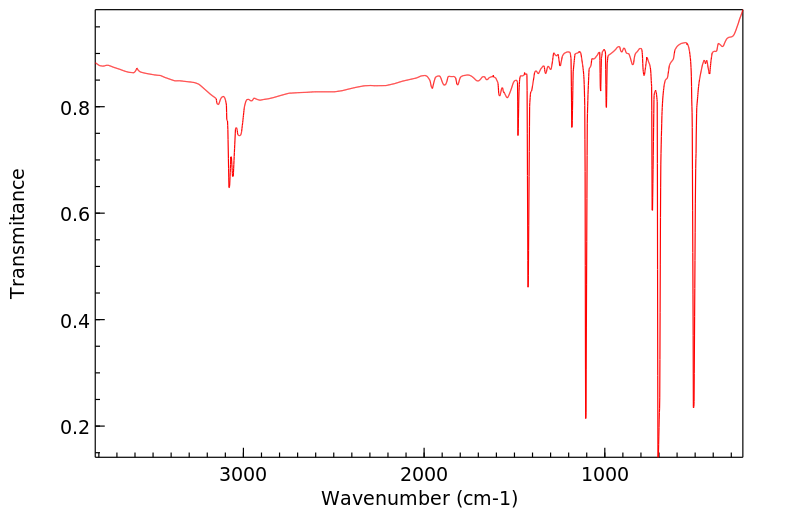
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫