(S)-2-氯-4-甲基戊酸 | 28659-81-6
中文名称
(S)-2-氯-4-甲基戊酸
中文别名
S-2-氯-4-甲基戊酸;(S)2-氯-4-甲基丙基乙酸;(S)-2-氯-4-甲基丙基乙酸
英文名称
(S)-2-chloro-4-methylvaleric acid
英文别名
(S)-2-chloro-4-methylpentanoic acid;(2S)-2-chloro-4-methylpentanoic acid
CAS
28659-81-6
化学式
C6H11ClO2
mdl
MFCD00044943
分子量
150.605
InChiKey
CBQBIPRPIHIKPW-YFKPBYRVSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:126 °C / 30mmHg
-
密度:1,09 g/cm3
-
稳定性/保质期:
如果按照规定使用和储存,则不会发生分解,目前没有已知的危险反应。
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:9
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.833
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险类别码:R36/37/38
-
海关编码:2915900090
-
安全说明:S26,S36/37/39
-
危险性防范说明:P210,P264,P280,P302+P352+P332+P313+P362+P364,P305+P351+P338+P337+P313,P370+P378,P403+P235,P501
-
危险性描述:H227,H315,H319
-
储存条件:请将贮藏器保持密封,并存放在阴凉、干燥处。同时,确保工作环境有良好的通风或排气设施。
SDS
(S)-2-氯-4-甲基戊酸 修改号码:5
模块 1. 化学品
产品名称: (S)-2-Chloro-4-methylvaleric Acid
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 可燃液体
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 远离明火/热表面。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
(S)-2-氯-4-甲基戊酸 修改号码:5
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): (S)-2-氯-4-甲基戊酸
百分比: >97.0%(T)
CAS编码: 28659-81-6
俗名: (S)-2-Chloro-4-methylpeNTanoic Acid
分子式: C6H11ClO2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
不适用的灭火剂: 棒状水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
(S)-2-氯-4-甲基戊酸 修改号码:5
模块 8. 接触控制和个体防护
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 126 °C/4kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.09
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氯化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
(S)-2-氯-4-甲基戊酸 修改号码:5
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: (S)-2-Chloro-4-methylvaleric Acid
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 可燃液体
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 远离明火/热表面。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
(S)-2-氯-4-甲基戊酸 修改号码:5
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): (S)-2-氯-4-甲基戊酸
百分比: >97.0%(T)
CAS编码: 28659-81-6
俗名: (S)-2-Chloro-4-methylpeNTanoic Acid
分子式: C6H11ClO2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
不适用的灭火剂: 棒状水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
(S)-2-氯-4-甲基戊酸 修改号码:5
模块 8. 接触控制和个体防护
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 126 °C/4kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.09
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氯化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
(S)-2-氯-4-甲基戊酸 修改号码:5
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Feichtinger,H., Chemische Berichte, 1962, vol. 95, p. 2238 - 2242摘要:DOI:
-
作为产物:参考文献:名称:由于与锌卟啉协同结合,α-卤代酰胺的不寻常构象摘要:α-卤素原子和羧酰胺基团与锌卟啉之间协同结合的 CD 和 NMR 光谱证据导致了前所未有的构象,用于测定 α-卤代酰胺(用 1,4-苯二胺衍生的 α-卤代羧酸)的绝对立体化学) 通过使用激子耦合圆二色性 (ECCD)。随着手性内酰胺的使用,其旋转异构贡献最小化,ECCD 和 NMR 光谱都表明,与较小的氢原子相比,卟啉更倾向于结合到空间上要求更高的卤素原子的一侧。总而言之,数据强烈暗示了α-手性酰胺以前未观察到的异常构象。提供了用于确定 α-卤代羧酸的绝对立体化学的助记符。 (© Wiley-VCH Verlag GmbH &DOI:10.1002/ejoc.200900089
文献信息
-
Design and synthesis of chromogenic thiopeptolide substrates as MetAPs active site probes作者:Yong-Mei Cui、Jing-Ya Li、Ling-Ling Chen、Jia Li、Qi-Zhuang Ye、Fa-Jun NanDOI:10.1016/j.bmc.2004.03.044日期:2004.6Twenty one chromogenic thiopeptolide substrates were designed and synthesized as the active site probes and analyzed with each S1 site of mutant residues and enzymes of wild-type MetAP1s. The preliminary enzymatic experiments indicate that cysteine 70 or 202, at either Escherichia coli or human MetAP1, played a crucial role in the methionine hydrolysis.
-
Umwandlung von Aldehyden in 2-monosubstituierte Aziridine. Reduktion von α-Chlor, Brom- und Sulfonyloxynitilen mit Lithiumaluminiumhydrid作者:Kunihiro Ichimura、Masaki OhtaDOI:10.1246/bcsj.43.1443日期:1970.5The reductive cyclization of α-chloro, bromo- and sulfonyloxycarbonitriles are described. α-Chlorocarbonitriles were reduced by lithium aluminum hydride to afford aziridines in sufficient yields. The Walden inversion was found to take place in the cource of the reductive cyclization of S-α-chloroisocapronitrile. We have found the two-step conversion of aldehydes to 2-mono-substituted aziridines which
-
Carboxylic Acid Compounds and Use Thereof申请人:Inoue Teruhiko公开号:US20070197512A1公开(公告)日:2007-08-23Provision of a superior URAT1 activity inhibitor effective for the treatment and the like of a pathology involving uric acid, such as hyperuricemia, gouty tophus, acute gouty arthritis, chronic gouty arthritis, gouty kidney, urinary lithiasis, renal dysfunction, coronary heart disease, ischemic cardiac diseases and the like. A URAT1 activity inhibitor containing a compound represented by the following formula [1] or a pharmaceutically acceptable salt thereof, or a solvate thereof as an active ingredient: wherein each symbol is as defined in the specification.
-
Synthesis of Muramyl Dipeptide Analogs with Enhanced Adjuvant Activity作者:Shigeru Kobayashi、Tsunehiko Fukuda、Hidefumi Yukimasa、Masahiko Fujino、Ichiro Azuma、Yuichi YamamuraDOI:10.1246/bcsj.53.2570日期:1980.9N-Acetylmuramyl-L-alanyl-D-isoglutamine (MDP) and thirteen new analogs were synthesized by the conventional organic chemical procedure using dicyclohexylcarbodiimide-N-hydroxy-5-norbornene-2,3-dicarboximide as a coupling agent. Their ability to induce delayed-type hypersensitivity to N-acetyl-3-(4-arsonophenylazo)-L-tyrosine in guinea pigs was assayed. The results indicate that the presence of an α-amino
-
Asymmetric Synthesis of 2-chloro- and 2-bromo-alkanoic acids by halogenation of α-D-glucofuranose-derived silyl ketene acetals作者:P Angibaud、J.L Chaumette、J.R Desmurs、L Duhamel、G Plé、J.Y Valnot、P DuhamelDOI:10.1016/0957-4166(95)00251-j日期:1995.82-chloro-alkanoic acids 6 and 7 have been obtained the diastereoselective halogenation of chiral silyl ketene acetals 3a-f, and subsequent saponification of the resulting crude esters. Examples characterized by e.e. values up to 95% are reported. The diastereoface selectivity is independent of the silyl ketene acetal configuration.
表征谱图
-
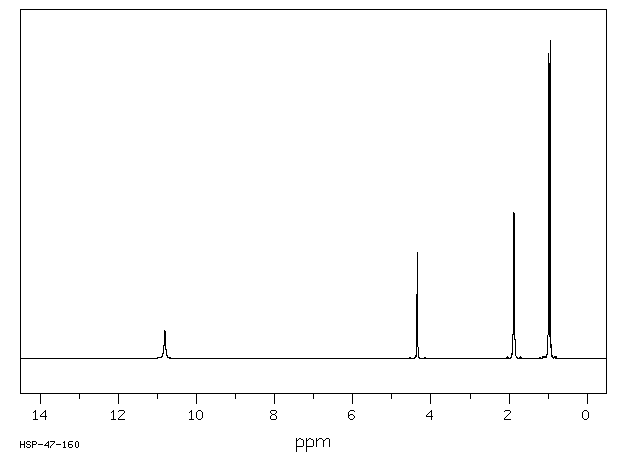
氢谱1HNMR
-
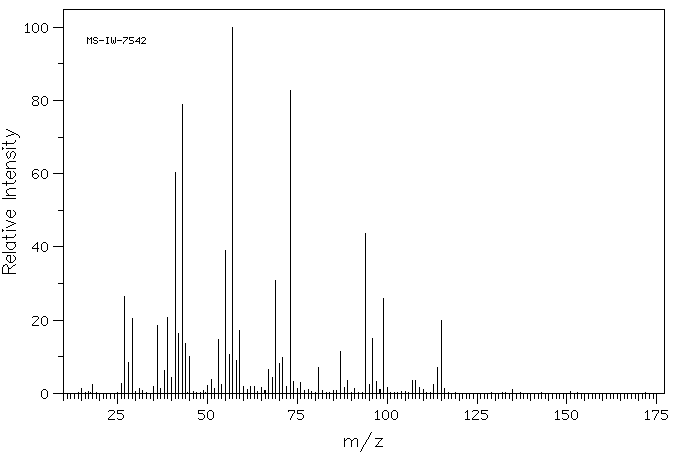
质谱MS
-
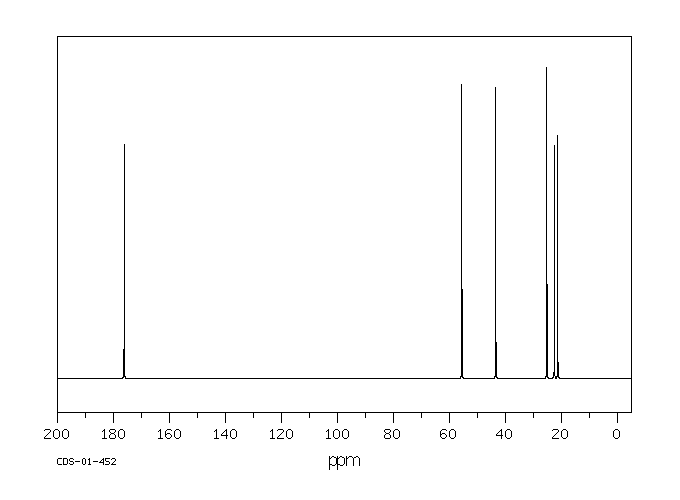
碳谱13CNMR
-
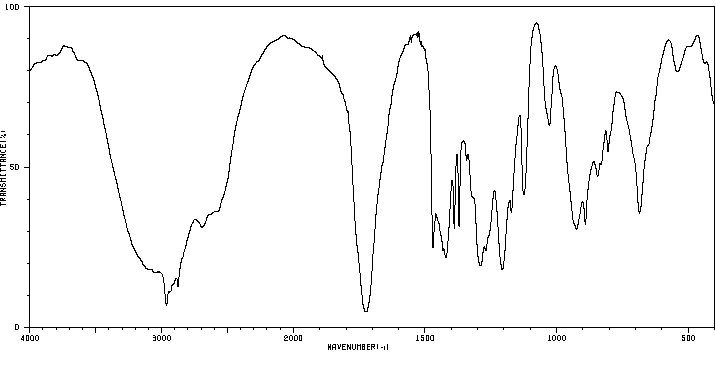
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯