10-甲基蒽-9-甲醛 | 7072-00-6
中文名称
10-甲基蒽-9-甲醛
中文别名
——
英文名称
10-methyl-9-anthraldehyde
英文别名
10-methylanthracene-9-carbaldehyde;10-methylanthracene-9-carboxaldehyde;9-methylanthracene-10-carbaldehyde
CAS
7072-00-6
化学式
C16H12O
mdl
——
分子量
220.271
InChiKey
KVWSVUPNZVIFBN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:169-171.5 °C (lit.)
-
沸点:321.21°C (rough estimate)
-
密度:1.0588 (rough estimate)
-
保留指数:383.1
计算性质
-
辛醇/水分配系数(LogP):4.2
-
重原子数:17
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.06
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
安全说明:S24/25
-
海关编码:2912299000
-
储存条件:| 2-8°C |
SDS
| Name: | 10-Methylanthracene-9-Carboxaldehyde Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 7072-00-6 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 7072-00-6 | 10-Methylanthracene-9-Carboxaldehyde | ca 100 | 230-366-9 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation. The toxicological properties of this material have not been fully investigated.
Skin:
May cause skin irritation. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 7072-00-6: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: gold
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 169.00 - 171.50 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C16H12O
Molecular Weight: 220.27
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 7072-00-6 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
10-Methylanthracene-9-Carboxaldehyde - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 7072-00-6: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 7072-00-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 7072-00-6 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 9-蒽甲醛 9-Anthraldehyde 642-31-9 C15H10O 206.244 9,10-二甲基蒽 9,10-dimethylanthracene 781-43-1 C16H14 206.287 9-甲基蒽 9-methylanthracene 779-02-2 C15H12 192.26 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 9,10-二甲基蒽 9,10-dimethylanthracene 781-43-1 C16H14 206.287 9-羟基甲基-10-甲基蒽 9-(hydroxymethyl)-10-dimethylanthracene 71339-55-4 C16H14O 222.287 —— 9-cyano-10-methylanthracene 1467-01-2 C16H11N 217.27 —— 10-formyl-9-methylanthracene oxime 119826-85-6 C16H13NO 235.285
反应信息
-
作为反应物:描述:参考文献:名称:Mancilla, Jerson M.; Nonhebel, Derek C.; Scullion, Ian, Journal of Chemical Research, Miniprint, 1980, # 3, p. 1601 - 1619摘要:DOI:
-
作为产物:描述:5-二苯并环庚烯酮 在 indium(III) triflate 、 二甲基二环氧乙烷 、 sodium sulfate 作用下, 以 四氢呋喃 、 乙醚 、 1,2-二氯乙烷 、 丙酮 为溶剂, 反应 36.0h, 生成 10-甲基蒽-9-甲醛参考文献:名称:通过酸催化环氧化物开环/半频哪醇重排从二苯并环庚醇环氧化物中取代 9-蒽醛摘要:以苯甲醛衍生物为原料,可以分五步制备相应的二苯并环庚烯醇。在两种底物(仲醇与叔醇和芳环上的取代基)和条件控制下,随后的环氧化和酸催化的环氧化物开环/半频哪醇重排/芳构化以良好的收率提供了相应的 9-蒽醛,高达 88 % 分两步。芳环上吸电子基团的存在抑制了环氧化速率,而随后的半频哪醇重排步骤需要加热;另一方面,给电子基团的存在经常导致环氧化过程中的分解。从机理研究来看,环氧化物的半频哪醇重排可以先于双苄基位置的电离,产生醛中间体。随后的脱水芳构化导致9-蒽醛的形成。相反,醛的亲核加成和脱水芳构化伴随着甲酸的损失导致蒽。DOI:10.1021/acs.joc.1c01405
文献信息
-
Native and modified chitosan-based hydrogels as green heterogeneous organocatalysts for imine-mediated Knoevenagel condensation作者:A. Franconetti、P. Domínguez-Rodríguez、D. Lara-García、R. Prado-Gotor、F. Cabrera-EscribanoDOI:10.1016/j.apcata.2016.03.012日期:2016.5Knoevenagel condensation catalysed with native and modified chitosan-based heterogeneous catalysts. The efficiency of our hydrogel organocatalysts, chitosan hydrogel beads and ureidyl-chitosan derivative hydrogel disks, was evaluated as function of pH, temperature and catalyst concentration by considering reaction rates, conversions, E/Z stereoselectivities, and kinetic studies of a model reaction between以天然和改性壳聚糖为基的多相催化剂,通过Knoevenagel缩合反应,合成了衍生自芳族和杂芳族醛的多种亚甲基丙二腈和氰基丙烯酸乙酯。考虑到反应速率,转化率,E / Z立体选择性以及模型间4 -硝基苯甲醛和氰基乙酸乙酯。固态的空前研究13当转化率达到50%后淬灭反应时,所用催化剂的C CP MAS NMR表明,在此过程中形成了亚胺-壳聚糖中间体。使用新颖的CLIP-HSQMCB实验,通过NMR测量碳-质子偶合常数(3 J C,H),进行E / Z氰基丙烯酸乙酯异构体混合物的分析,以确定相应的立体选择性。此外,DFT计算使我们合理化了所观察到的E / Z立体选择性,并评估了脲基部分对与醛相互作用和与壳聚糖衍生物形成亚胺中间体的作用。
-
Tryptamine-Based Derivatives as Transient Receptor Potential Melastatin Type 8 (TRPM8) Channel Modulators作者:Alessia Bertamino、Carmine Ostacolo、Paolo Ambrosino、Simona Musella、Veronica Di Sarno、Tania Ciaglia、Maria Virginia Soldovieri、Nunzio Iraci、Asia Fernandez Carvajal、Roberto de la Torre-Martinez、Antonio Ferrer-Montiel、Rosario Gonzalez Muniz、Ettore Novellino、Maurizio Taglialatela、Pietro Campiglia、Isabel Gomez-MonterreyDOI:10.1021/acs.jmedchem.5b01914日期:2016.3.10Pharmacological modulation of the transient receptor potential melastatin type 8 (TRPM8) is currently under investigation as a new approach for the treatment of pain and other diseases. In this study, a series of N-substituted tryptamines was prepared to explore the structural requirements determining TRPM8 modulation. Using a fluorescence-based screening assay, we identified two compounds acting as目前正在研究8型瞬时受体电位褪黑素(TRPM8)的药理学调节,作为治疗疼痛和其他疾病的新方法。在这项研究中,准备了一系列的N-取代的色胺,以探索确定TRPM8调节的结构要求。使用基于荧光的筛选分析,我们确定了两种化合物作为激活剂(2-(1 H-吲哚-3-基)-N-(4-苯氧基苄基)乙胺,21)或抑制剂(N,N-二苄基-2-(1 H-吲哚-3-基)乙胺,12)的钙流入HEK293细胞。在膜片钳录音中,化合物21与薄荷醇相比,显示出显着更高的效力(EC 50 = 40±4μM)和相似的功效;相反,化合物12对薄荷醇诱导的TRPM8电流产生浓度依赖性抑制(IC 50 = 367±24 nM)。使用单个大鼠TRPM8亚基的同源性模型进行的分子建模研究确定了位于VSD和TRP盒之间的推定结合位点,揭示了激动剂和拮抗剂的结合方式不同。
-
一种茂型钌配合物的制备方法及其抗肿瘤应用申请人:云南大学公开号:CN109232670A公开(公告)日:2019-01-18
-
Catalytic Enantioselective Allyl- and Crotylboration of Aldehydes Using Chiral Diol•SnCl<sub>4</sub> Complexes. Optimization, Substrate Scope and Mechanistic Investigations作者:Vivek Rauniyar、Huimin Zhai、Dennis G. HallDOI:10.1021/ja8016076日期:2008.7.1induction in the allylboration of aldehydes by commercially available allylboronic acid pinacol ester 1a. The corresponding homoallylic alcohol products of synthetically useful aliphatic aldehydes are obtained in excellent yields with up to 98:2 er. This combined acid manifold is also efficient in catalyzing the diastereo- and enantioselective crotylboration of aldehydes, thus providing the propionate我们报告了一类新的 C2 对称手性二醇,其源自对苯二酚骨架。在 Yamamoto 的路易斯酸辅助布朗斯台德酸度(LBA 催化)概念下,这些二醇与 SnCl 4 的组合导致可商购的烯丙基硼酸频哪醇酯 1a 在醛的烯丙基硼化中产生高水平的不对称诱导。合成有用的脂肪醛的相应高烯丙醇产品以高达 98:2 的产率获得。这种组合的酸歧管还可有效催化醛的非对映选择性和对映选择性巴豆基硼化反应,从而提供 >95:5 dr 和高达 98:2 er 的丙酸酯单元。最佳二醇 x SnCl4 配合物 Vivol (4m) x SnCl4 的 X 射线晶体结构,明确显示了这种 LBA 催化剂的 Brønsted 酸性特征及其高度不对称的环境。进一步的控制排除了可能的硼与手性二醇的酯交换机制,并指出频哪醇烯丙基硼酸酯 1 的 LBA 催化剂衍生的活化。由于二醇 x SnCl4 复合物的缓慢解离,需要少量过量的二醇为了抑制由游离
-
Spin-Allowed Transitions Control the Formation of Triplet Excited States in Orthogonal Donor-Acceptor Dyads作者:Jason T. Buck、Andrew M. Boudreau、André DeCarmine、Reid W. Wilson、James Hampsey、Tomoyasu ManiDOI:10.1016/j.chempr.2018.10.001日期:2019.1Triplet excited states (triplets) serve as key intermediates in critical technologies and processes ranging from organic synthesis to biomedicine to molecular electronics. Production of triplets of π-conjugated organic molecules without heavy atoms remains challenging. Spin-orbit, charge-transfer intersystem crossing (SOCT-ISC) directly converts singlet charge-separated states to triplets in an electron三重态激发态(三重态)是从有机合成到生物医学再到分子电子学等关键技术和过程的关键中间体。没有重原子的三重π共轭有机分子的生产仍然具有挑战性。自旋轨道,电荷转移系统间交叉(SOCT-ISC)将单重态电荷分离态直接转换为电子供体-受体(DA)对中的三重态。在这里,使用一系列正交的DA型硼二吡咯亚甲基(BODIPY)衍生物作为模型系统,我们表明三联体的形成很大程度上受自旋允许的跃迁控制,而不是受SOCT-ISC的控制。然而,在(π,π*)状态之间,SOCT-ISC过程仍比普通ISC进行得快得多,这是因为SOCT-ISC的自旋轨道耦合强了2个数量级。我们进一步表明,这样的过程可以在非三重峰形成分子per中产生三重峰。我们的发现揭示了禁止自旋过程的明确物理基础,并为利用该过程的未来分子设计提供了指导。
表征谱图
-
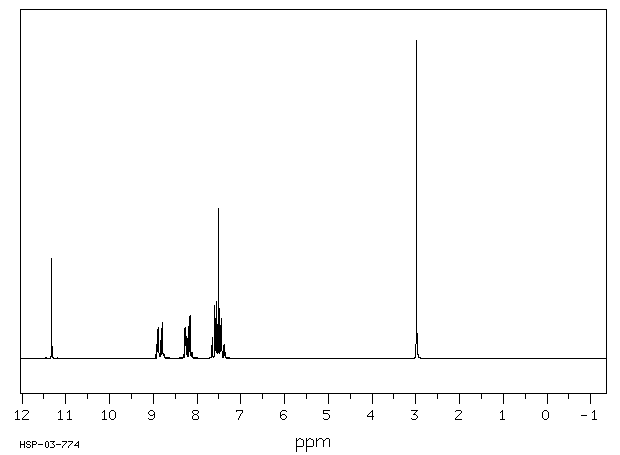
氢谱1HNMR
-
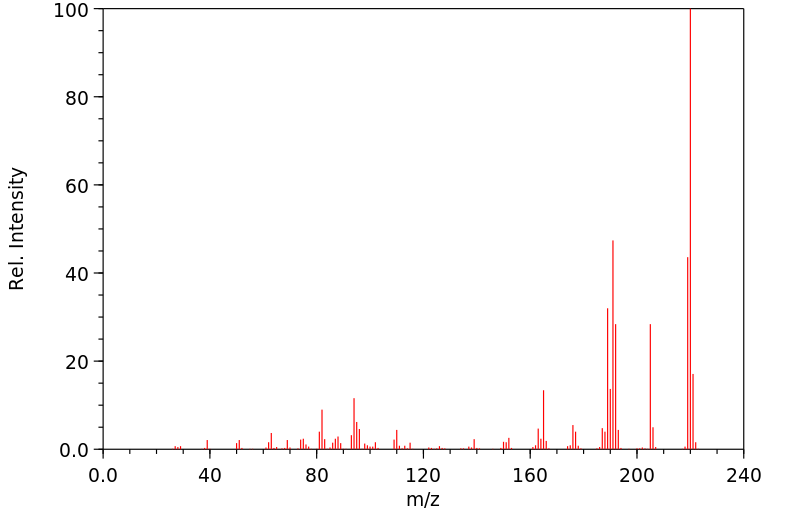
质谱MS
-
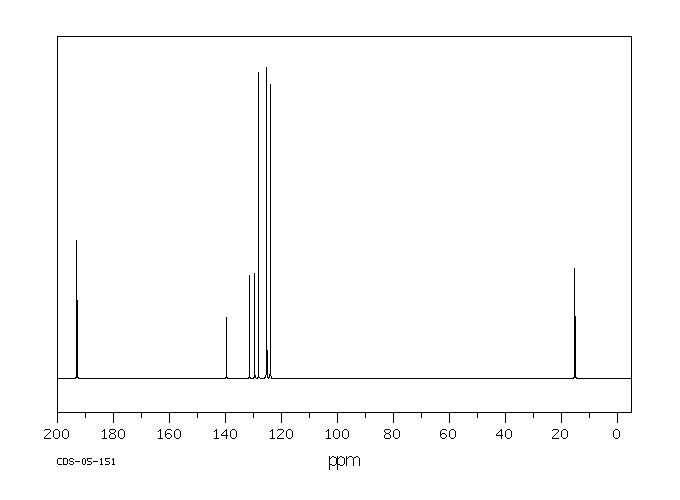
碳谱13CNMR
-
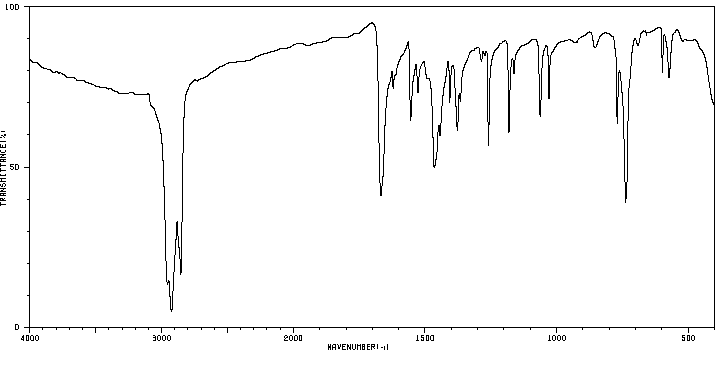
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐斯托醌
黄决明素
马普替林相关物质D
马普替林杂质E(N-甲基马普替林)
马普替林杂质D
马普替林D3
马普替林
颜料黄199
颜料黄147
颜料黄123
颜料黄108
颜料红89
颜料红85
颜料红251
颜料红177
颜料紫27
顺式-1-(9-蒽基)-2-硝基乙烯
阿美蒽醌
阳离子蓝FGL
阳离子蓝3RL
长蠕孢素
镁蒽四氢呋喃络合物
镁蒽
锈色洋地黄醌醇
锂钠2-[[4-[[3-[(4-氨基-9,10-二氧代-3-磺基-1-蒽基)氨基]-2,2-二甲基-丙基]氨基]-6-氯-1,3,5-三嗪-2-基]氨基]苯-1,4-二磺酸酯
锂胭脂红
链蠕孢素
铷离子载体I
铝洋红
铂(2+)二氯化1-({2-[(2-氨基乙基)氨基]乙基}氨基)蒽-9,10-二酮(1:1)
钾6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠alpha-(丙烯酰氨基)-[4-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]苯氧基]甲苯磺酸盐
钠[[3-[[4-(环己基氨基)-9,10-二氢-9,10-二氧代-1-蒽基]氨基]-1-氧代丙基]氨基]苯磺酸盐
钠[3-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]丁基]苯磺酸盐
钠6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠4-({4-[乙酰基(乙基)氨基]苯基}氨基)-1-氨基-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠2-[(4-氨基-9,10-二氧代-3-磺基-9,10-二氢-1-蒽基)氨基]-4-{[2-(磺基氧基)乙基]磺酰基}苯甲酸酯
钠1-氨基-9,10-二氢-4-[[4-(1,1-二甲基乙基)-2-甲基苯基]氨基]-9,10-二氧代蒽-2-磺酸盐
钠1-氨基-4-[(3-{[(4-甲基苯基)磺酰基]氨基}苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-[(3,4-二甲基苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-(1,3-苯并噻唑-2-基硫基)-9,10-二氧代蒽-2-磺酸盐
醌茜隐色体
醌茜素
酸性蓝P-RLS
酸性蓝41
酸性蓝27
酸性蓝127:1
酸性紫48
酸性紫43
酸性兰62