ethyl 3-nonenoate | 91213-30-8
中文名称
——
中文别名
——
英文名称
ethyl 3-nonenoate
英文别名
3-nonenoic acid ethyl ester;ethyl non-3-enoate
CAS
91213-30-8
化学式
C11H20O2
mdl
——
分子量
184.279
InChiKey
KQCNXVHFHZAHDW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:13
-
可旋转键数:8
-
环数:0.0
-
sp3杂化的碳原子比例:0.73
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
反应信息
-
作为反应物:描述:ethyl 3-nonenoate 、 间氯过氧苯甲酸 在 氢氧化钙 、 Calcium o-chlorobenzoate 、 二氯甲烷 、 Sodium sulfate-III 作用下, 以 二氯甲烷 为溶剂, 反应 17.5h, 以24.6 g of crude 3,4-epoxynonanoic acid ethyl ester were obtained (93% pure according to GC)的产率得到3,4-Epoxynonanoic acid ethyl ester参考文献:名称:Novel Use of Nonenolide摘要:本发明涉及使用非烯酮来赋予、改变和/或增强一种或多种气味或口味印象,所述气味或口味印象来自于草、香豆素、内酯和口感丰富度的组合。公开号:US20070297993A1
-
作为产物:描述:乙醇 、 1-辛烯-3-醇乙酸酯 、 一氧化碳 在 tris(dibenzylideneacetone)dipalladium(0) chloroform complex N,N-二异丙基乙胺 、 三苯基膦 、 sodium bromide 作用下, 50.0 ℃ 、3.04 MPa 条件下, 反应 20.0h, 以83%的产率得到ethyl 3-nonenoate参考文献:名称:Palladium(0)-catalyzed alkoxycarbonylation of allyl phosphates and acetates摘要:Palladium-catalyzed alkoxycarbonylation of allyl phosphates under CO (1 atm) at 50-degrees-C proceeds highly efficiently to give the corresponding beta,gamma-unsaturated esters. The carbonylation of geranyl phosphate ((E)-11) under CO (1 atm) at 50-degrees-C gave ethyl ester of homogeranic acid ((E)-12) stereoselectively. The carbonylation takes place at the least substituted allylic positions with inversion of configuration. Typically, the methoxycarbonylation of cis-5-(methoxycarbonyl)-2-cyclohexen-1-yl phosphate (cis-16) gave trans-dimethyl 2-cyclohexene-1,5-dicarboxylate (trans-17) selectively. Alkoxycarbonylation of allyl acetates is performed for the first time in the presence of a catalytic amount of bromide ion. The reaction can be rationalized by assuming the mechanism which involves oxidative addition of palladium(0) species to allyl acetates to give pi-allylpalladium acetate, fast ligand exchange of the acetate with bromide, insertion of carbon monoxide to give acylpalladium species, and alkoxylation.DOI:10.1021/jo00058a040
文献信息
-
A Convenient Synthesis of β,γ-Unsaturated Carboxylic Acid Derivatives via Carbonylation of (η<sup>3</sup>-Allyl)Fe(CO<sub>2</sub>NO Complexes作者:Saburo Nakanishi、Takeshi Yamamoto、Naoyuki Furukawa、Yoshio OtsujiDOI:10.1055/s-1994-25533日期:——One-pot reaction of allyl halides with [Bu4N]+[Fe(CO)3(NO)]- followed by treatment with 1,2-bis(diphenylphosphino)ethane gave βγ-unsaturated acyl iron complexes in good yields via regioselective carbonylation with retention of configuration of the allylic double bond. β,γ-Unsaturated carboxylic acid esters and amides were prepared upon treatment of the acyl iron complexes with alcohols and amines in the presence of iodine.
表征谱图
-
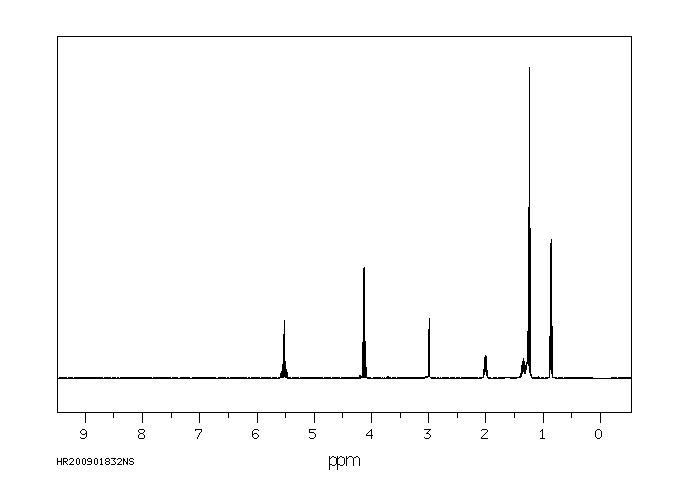
氢谱1HNMR
-
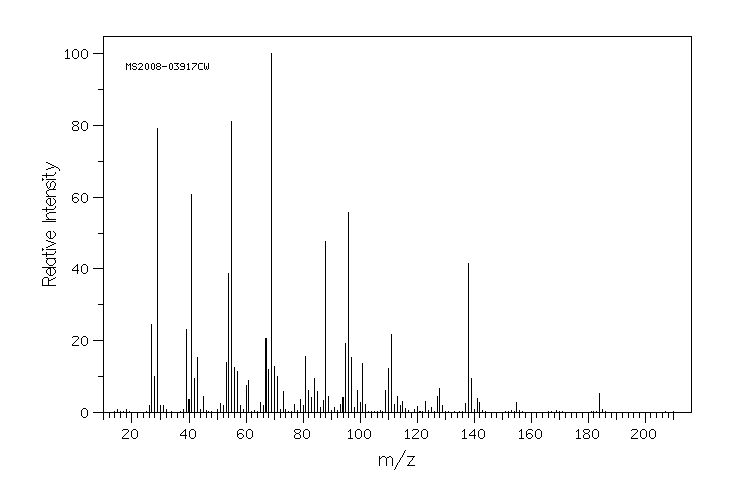
质谱MS
-
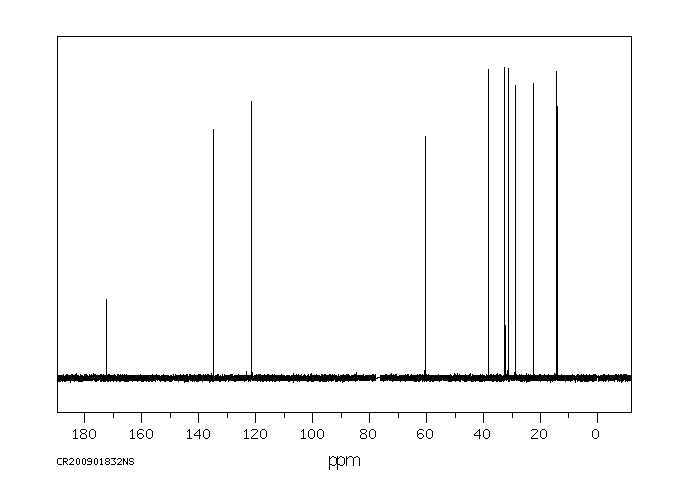
碳谱13CNMR
-
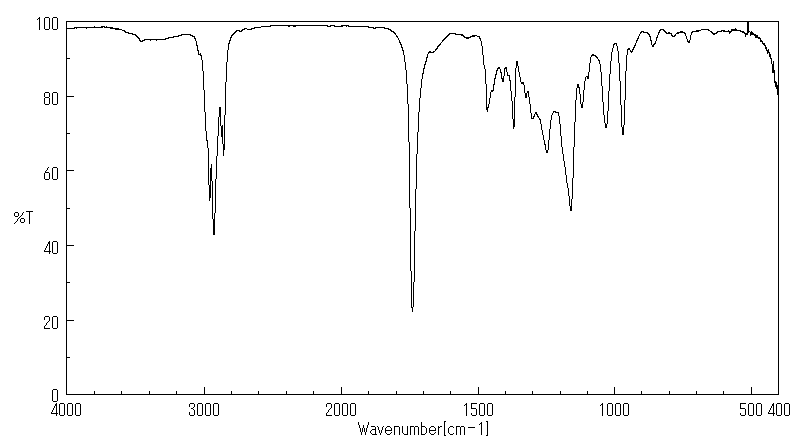
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯