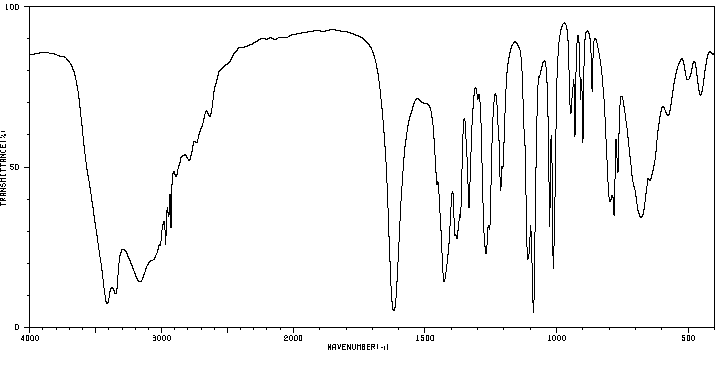
代谢
Calcium gluconate does not require hepatic metabolism for the release of Ca++ and is as effective as calcium chloride in treating ionic hypocalcemia in the absence of hepatic function.
来源:DrugBank