[4-(苯甲酰基)-1,2,5-恶二唑-3-基]-苯基甲酮 | 10349-12-9
中文名称
[4-(苯甲酰基)-1,2,5-恶二唑-3-基]-苯基甲酮
中文别名
——
英文名称
3,4-Dibenzoyl-1,2,5-oxadiazole
英文别名
C,C'-diphenyl-C,C'-furazan-3,4-diyl-bis-methanone;3,4-dibenzoyl-furazan;Dibenzoyl-furazan;3,4-Dibenzoyl-1,2,5-oxadiazol;3,4-Dibenzoyl-furoxan;Furazan, dibenzoyl-;(4-benzoyl-1,2,5-oxadiazol-3-yl)-phenylmethanone
CAS
10349-12-9
化学式
C16H10N2O3
mdl
——
分子量
278.267
InChiKey
NPRKTQDWJWXWET-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:118 °C
-
沸点:491.7±55.0 °C(Predicted)
-
密度:1.294±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:21
-
可旋转键数:4
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:73.1
-
氢给体数:0
-
氢受体数:5
安全信息
-
海关编码:2934999090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 alpha,alpha'-[(1,2,5-恶二唑5-氧化物)-3,4-二基]二苯甲醛 3,4-dibenzoyl-1,2,5-oxadiazole-N-oxide 6635-54-7 C16H10N2O4 294.266
反应信息
-
作为反应物:描述:[4-(苯甲酰基)-1,2,5-恶二唑-3-基]-苯基甲酮 在 sodium tetrahydroborate 作用下, 以 乙醇 、 正丁醇 为溶剂, 反应 36.0h, 生成 3,4-二氨基-2,5-二苯基-6-羟甲基吡啶参考文献:名称:ALKOXYSILYL GROUP-CONTAINING ORGANIC EL DYE AND A METHOD FOR PRODUCING THE SAME摘要:提供一种含有烷氧基硅基团的有机EL染料,可用于制备不会褪色的荧光二氧化硅微粒。这种含有烷氧基硅基团的有机EL染料由通式X—Y-Q-Z—Si(R1)n(OR2)3-n表示,其中X是有机EL染料,Y是直接键或—(CH2)p—(p代表1到10的整数)或—(O—CH2CH2)q—(q代表1到10的整数),Q是选择自酰胺键、醚键、硫醚键、硫酯键、硫脲键、二硫键和聚氧乙烯键的一种键,Z是—(CH2)3—或—(CH2)2NH(CH2)3—,R1和R2代表具有1至7个碳原子的烷基,n代表0或1。公开号:US20170313885A1
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 在 盐酸 、 溶剂黄146 、 tin(ll) chloride 作用下, 生成 [4-(苯甲酰基)-1,2,5-恶二唑-3-基]-苯基甲酮参考文献:名称:de Paolini, Gazzetta Chimica Italiana, 1927, vol. 57, p. 658摘要:DOI:
文献信息
-
一种3,4-二苯甲酮-1,2,5-恶二唑的制备方法
-
FLUORESCENT DYE申请人:Isobe Shinichiro公开号:US20140206872A1公开(公告)日:2014-07-24There is provided a fluorescent dye which can improve the fluorescent intensity at the time of labeling to thereby detect a biomolecule with higher sensitivity. The fluorescent dye of the present invention includes a nitrogen cation-containing group or a nitrogen-containing group. Its high water solubility can improve the rate of labeling for a biomolecule to thereby detect a biomolecule with high sensitivity.
-
10-Hydroxy-7-arylindeno[1,2-b]-1,2,5-oxadiazolo[3,4-d]pyridines and 7-Aryl-10-oxoindeno[1,2-b]-1,2,5-oxadiazolo[3,4-d]pyridines— Synthesis, Spectra, and Polymorphism作者:Shuntaro Mataka、Hideki Gorohmaru、Thies Thiemann、Tsuyoshi Sawada、Kazufumi Takahashi、Akiyoshi Tori-iDOI:10.3987/com-98-s(h)88日期:——The novel dyes 7-aryl-10-oxoindeno[1,2-b]-1,2,5-oxadiazolo[3,4-d]pyridines (4) and 7-aryl-10-hydroxyindeno[1,2-b]-1,2,5-oxadiazolo[3,4-d]pyridines (5) have been prepared from acetophenone derivatives. While compounds (4) exhibit a dark red color, they are only weakly fluorescent Compounds (5) is fluorescent. Of interest is that 10-hydroxy-7-phenylindeno-[1,2-b]-1,2,5-oxadiazolo[3,4-d] pyridine (5a) can take four polymorphic forms in the solid state, of which two are yellow (designated as 5a-Y-1 and 5a-Y-2) and two are red (5a-R-1 and 5a-R-2). Two of them are interconvertible (yellow/red) upon exposure to different solvents. X-Ray crystal structure analysis of 5a-R-2 shows the phenyl ring and the indenooxadiazolopyridine ring to be coplanar.
-
Angeli, Chemische Berichte, 1893, vol. 26, p. 529作者:AngeliDOI:——日期:——
-
Reaction of 3,4-Diaroyl-1,2,5-thia-(or -oxa)-diazoles and<i>o</i>-Dibenzoylbenzene with Mineral Acid Salts of Methylamines having an Electron-Withdrawing Group作者:Shuntaro Mataka、Kazufumi Takahashi、Masashi Tashiro、Yuhsuke TsudaDOI:10.1055/s-1980-29231日期:——
表征谱图
-
氢谱1HNMR
-
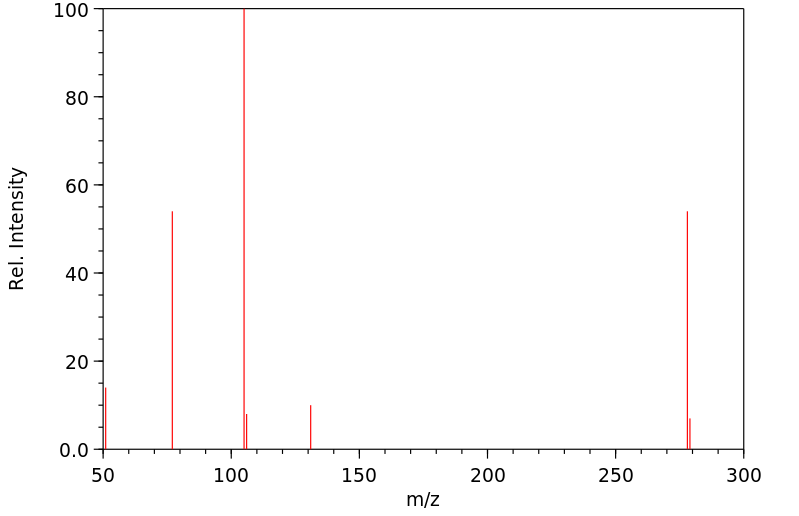
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷