三醋精 | 102-76-1
中文名称
三醋精
中文别名
三醋酸甘油酯;甘油三乙酸酯;丙三醇三乙酸酯;三乙酸甘油酯;醋精;乙酸甘油酯;甘油醋酸酯;三乙酸丙三醇酯;1,2,3-丙三醇三乙酸酯;三醋汀
英文名称
triacetylglycerol
英文别名
triacetin;glyceryl triacetate;glycerol triacetate;1,2,3-propanetriol triacetate;2,3-diacetyloxypropyl acetate
CAS
102-76-1
化学式
C9H14O6
mdl
MFCD00008716
分子量
218.207
InChiKey
URAYPUMNDPQOKB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:3 °C(lit.)
-
沸点:258-260 °C(lit.)
-
密度:1.16 g/mL at 25 °C(lit.)
-
蒸气密度:7.52 (vs air)
-
闪点:300 °F
-
溶解度:易溶于水,与乙醇(96%)和甲苯混溶。
-
介电常数:7.2(20℃)
-
LogP:0.25
-
物理描述:Liquid
-
颜色/状态:Colorless liquid
-
气味:Slightly fatty odor
-
味道:MILD, SWEET TASTE, BITTER ABOVE 0.05%
-
蒸汽密度:7.52 (Air = 1)
-
蒸汽压力:0.00248 mm Hg at 25 °C
-
稳定性/保质期:
请确保不与氧化剂接触。
-
自燃温度:812 °F (433 °C)
-
分解:When heated to decomposition it emits acrid smoke and irritating fumes.
-
折光率:Index of refraction = 1.4312 at 20 °C/D
-
保留指数:1306;1285;1282;1348;1305;1308;1310;1313;1305.9;1282
计算性质
-
辛醇/水分配系数(LogP):0.2
-
重原子数:15
-
可旋转键数:8
-
环数:0.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:78.9
-
氢给体数:0
-
氢受体数:6
ADMET
代谢
Triacetin has been administered iv to mongrel dogs. The majority of infused triacetin underwent intravascular hydrolysis, and the majority of the resulting acetate is oxidized. Triacetin was found to be hydrolyzed by human intestinal lipase.
来源:Hazardous Substances Data Bank (HSDB)
代谢
...Triacetin is rapidly hydrolysed in vitro by all tissues of the organism including the gastrointestinal tract.
来源:Hazardous Substances Data Bank (HSDB)
代谢
一组雌性混种狗用于研究等热量和高热量输注5% v/v水溶性三醋酸甘油对代谢的影响。首先,持续输注5微摩尔/千克(0.3微居里/千克/分钟)的[(13)C]-乙酰乙酸盐和1.0微居里/千克(0.01微居里/千克/分钟)的[(3)H]-葡萄糖,持续6小时。同位素输注开始后3小时,开始输注三醋酸甘油。六只动物以47微摩尔/千克/分钟的速度输注,七只动物以70微摩尔/千克/分钟的速度输注三醋酸甘油,持续3小时。每隔15到30分钟采集血液和呼吸样本。一组四只动物以70微摩尔/千克/分钟的速度输注甘油,用作高热量输注的对照。在等热量输注三醋酸甘油期间,血浆醋酸和自由脂肪酸浓度在30分钟和60分钟分别显著增加,并保持升高。在高热量输注期间,血浆醋酸浓度在整个研究过程中逐渐增加,而血浆自由脂肪酸浓度没有变化。血浆丙酮酸和乳酸浓度在30分钟和90分钟分别显著降低,并且在等热量和高热量输注期间始终降低。血浆胰岛素浓度在两种输注期间适度增加。血浆葡萄糖浓度在等热量三醋酸甘油输注期间显著降低;在高热量输注期间观察到轻微但显著的升高。在输注的最后 hour,两组的葡萄糖清除率显著降低。血浆酮体浓度在60分钟时显著增加,并且在等热量输注期间保持升高,在高热量输注三醋酸甘油期间逐渐增加;增加的浓度是由于酮体产生增加。在输注的最后 hour,等热量三醋酸甘油的静息能量消耗显著增加。
Groups of female mongrel dogs to study the metabolic effects of isocaloric and hypercaloric infusions of 5% v/v aqueous triacetin. A primed, continuous infusion of 5 umol/kg (0.3 uCi/kg/min) [(13)C]-acetoacetate and 1.0 uCi/kg (0.01 uCi/kg/min) [(3)H]-glucose was continued for 6 hr. Three hours after the start of the isotope infusion, dosing with triacetin was started. Six animals were infused at a rate of 47 umol/kg/min and seven were infused at a rate of 70 umol/kg/min triacetin for 3 hr. Blood and breath samples were taken at 15 to 30-min intervals. A group of four animals was infused with 70 umol/kg/min glycerol and used as the control for the hypercaloric infusion. During isocaloric infusion of triacetin, plasma acetate and free fatty acid concentrations were significantly increased at 30 and 60 min, respectively, and remained elevated. During hypercaloric infusion, plasma acetate concentration increased progressively throughout the study, whereas the plasma free fatty acid concentration did not change. Plasma pyruvate and lactate concentrations were significantly decreased after 30 and 90 min, respectively, and throughout the study with both isocaloric and hypercaloric infusion. The plasma insulin concentrations were modestly increased during both infusions. Plasma glucose concentration was significantly decreased during isocaloric triacetin infusion; a slight but significant increase was observed with hypercaloric infusion. Glucose clearance decreased significantly in both groups during the last hour of triacetin infusion. Plasma ketone body concentrations increased significantly by 60 min, and they remained elevated with isocaloric infusion and increased progressively with hypercaloric infusion of triacetin; the increased concentrations were due to increased ketone body production. During the last hour of infusion, resting energy expenditure was significantly increased with isocaloric triacetin.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Esterases in fungi or in serum act at pH >4 to slowly release acetic acid in situ. Extent of hydrolysis is automatically limited by increased acidity and lowering of pH.
来源:Hazardous Substances Data Bank (HSDB)
代谢
... The fungistatic activity of triacetin (glyceryl triacetate) results from its hydrolysis by fungalesterases to acetic acid.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
识别和使用:三醋精,也称为甘油三醋酸(GTA),是一种无色液体。据报道,它在化妆品配方中可作为化妆品杀菌剂、塑化剂和溶剂使用。三醋精还用作制造香烟过滤器的纤维素塑化剂,作为杀菌剂组合物中的载体,以及从天然气中去除二氧化碳。三醋精被美国食品药品监督管理局确认为一般认为安全的(GRAS)人类食品成分。三醋精曾用于实验性治疗卡纳万病,这是一种致命的脱髓鞘遗传障碍,与天冬酰乙酰酶缺乏有关,导致发育中的大脑乙酸盐水平降低和髓磷脂合成减少。人类暴露和毒性:在人类中,商用三醋精曾引起眼部刺激,但未造成伤害。在一项临床最大化研究中,三醋精不是刺激剂也不是致敏剂,而在杜尔林室试验中使用50%稀释液时,只观察到非常轻微的反应。在敏感个体中,它可能引起轻微刺激。动物研究:三醋精通过腹腔、皮下和静脉途径给药具有中等毒性。三醋精的毒性症状主要是虚弱和共济失调。濒临死亡的动物会出现严重的呼吸困难、肌肉震颤和偶尔的抽搐,通常在注射后2-22分钟内。还观察到不同程度的肺部出血。它似乎不影响肝脏、脾脏、心脏或肾脏。在短期喂养研究中,三醋精影响了体重增加。被喂食含有30%三醋精作为淀粉替代品3至4周或12至13周的饮食的大鼠,生长相对较差。所有动物都观察到肝脏肿大。在短期研究中,通过吸入或经皮给药的三醋精不具有毒性,在亚慢性研究中,通过饲料或吸入给药的三醋精也不具有毒性。三醋精在豚鼠皮肤上最多只有轻微的刺激性。然而,在一项研究中,它引起了红斑、轻微水肿、脱发和脱屑。三醋精在豚鼠中不具有致敏性。三醋精对兔眼有一定的刺激性。在一项对狗的研究中,发现胃内输注1.0%-2.0%的三醋精通过增加近端胃接受容量,暂时抑制胃窦收缩并促进十二指肠收缩,从而延迟胃排空。在一项使用三醋精作为向遭受创伤性脑损伤的大鼠大脑输送可代谢乙酸盐的方法的研究中,发现三醋精给药在损伤后4天和6天显著提高了损伤半球NAA(N-乙酰天冬氨酸)和ATP的水平,并在损伤后3天显著改善了大鼠的motor性能。三醋精在有或没有代谢激活的情况下,在使用鼠伤寒沙门氏菌TA98、TA100、TA1535、TA1537菌株的埃姆斯试验中不具有致突变性。在利用果蝇进行的体内试验中,它也不具有致突变性。
IDENTIFICATION AND USE: Triacetin, also known as glyceryl triacetate (GTA), is a colorless liquid. It is reported to function as a cosmetic biocide, plasticizer, and solvent in cosmetic formulations. Triacetin is also used as a cellulose plasticizer in the manufacture of cigarette filters, as a carrier in fungicidal compositions, and to remove carbon dioxide from natural gas. Triacetin was affirmed as a GRAS (generally recognized as safe) human food ingredient by FDA. Triacetin has been used experimentally for the treatment of Canavan disease, a fatal dysmyelinating genetic disorder associated with aspartoacylase deficiency, resulting in decreased brain acetate levels and reduced myelin lipid synthesis in the developing brain. HUMAN EXPOSURE AND TOXICITY: In humans, commercial Triacetin has caused ocular irritation but no injury. Triacetin was not an irritant or a sensitizer in a clinical maximization study, and only very mild reactions were seen in a Duhring-chamber test using a 50% dilution. In sensitive individuals, it may cause slight irritation. ANIMAL STUDIES: Triacetin is moderately toxic by intraperitoneal, subcutaneous, and intravenous routes. The symptoms of toxicity with triacetin are primarily weakness and ataxia. Animals near death exhibit severe dyspnea, muscular tremors, and occasional convulsions, usually 2-22 min after the injection. Also various degrees of hemorrhage in the lung have been observed. It does not appear to affect the liver, spleen, heart, or kidneys. In short-term feeding studies, triacetin affected weight gain. Rats that were fed diets containing 30% triacetin as a starch substitute for 3 to 4 or 12 to 13 weeks, showed relatively poor growth. Liver enlargement was observed in all animals. Triacetin was not toxic in short-term studies when administered via inhalation or parenterally or in subchronic studies when administered via feed or inhalation. Triacetin was, at most, slightly irritating to guinea pig skin. However, in one study, it caused erythema, slight edema, alopecia, and desquamation. Triacetin was not sensitizing in guinea pigs. Triacetin caused some irritation in rabbit eyes. In a study performed on dogs, it was found that intra-gastric infusion of 1.0%-2.0% triacetin delays gastric emptying by increasing proximal stomach receptive volume, temporarily inhibiting gastric antral contractions and facilitating duodenal contractions. In a study using triacetin as a method of delivering metabolizable acetate to the brain of rats suffering traumatic brain injury, it was found that triacetin administration significantly increased the levels of both NAA (N-acetylaspartate) and ATP in the injured hemisphere 4 and 6 days after injury, and also resulted in significantly improved motor performance in rats 3 days after injury. Triacetin, with and without metabolic activation, was not mutagenic in the Ames assay using Salmonella typhimurium strains TA98, TA100, TA1535, TA153 with or without activation. It was also not mutagenic in an in vivo assay using Drosophila.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
Redness.
Redness.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
神经毒素 - 其他中枢神经系统神经毒素
Neurotoxin - Other CNS neurotoxin
来源:Haz-Map, Information on Hazardous Chemicals and Occupational Diseases
毒理性
LC50 (大鼠) > 1,721 毫克/立方米/4小时
LC50 (rat) > 1,721 mg/m3/4h
来源:Haz-Map, Information on Hazardous Chemicals and Occupational Diseases
毒理性
当前研究调查了静脉注射短链甘油三酯丙三醇对狗体内亮氨酸代谢的影响。动物在[(1-14)C]亮氨酸输注期间分别接受了1.0倍预估静息能量消耗(REE)的丙三醇、1.5倍REE的超能丙三醇、甘油或生理盐水的输注。在两次丙三醇输注期间,血浆α-酮异己酸浓度增加(p < 0.05)。在1.5倍REE的丙三醇输注期间,血浆亮氨酸浓度降低(p < 0.05),亮氨酸出现速率降低了大约19%(p < 0.05);这一变化显著大于1.0倍REE丙三醇和甘油时的变化(p < 0.05)。在1.0倍REE丙三醇输注的狗和对照组之间,亮氨酸的氧化没有差异,而在1.5倍REE丙三醇输注期间,亮氨酸的氧化减少了53%(p < 0.05)。非氧化性亮氨酸消失,即蛋白质合成的指标,在任何研究中都没有变化。
The present studies investigated the effects of intravenous administration of the short-chain triglyceride triacetin on leucine metabolism in dogs. Animals received infusions of triacetin at 1.0 x estimated resting energy expenditure (REE), hyperenergetic triacetin at 1.5 x REE, glycerol, or saline during infusion of [(1-14)C]leucine. During both triacetin infusions, plasma alpha-ketoisocaproate concentrations increased (p < 0.05). During triacetin infusion at 1.5 REE, the plasma leucine concentration decreased (p < 0.05) and leucine rate of appearance decreased by approximately 19% (p < 0.05); this was significantly greater than the changes that occurred during triacetin at 1.0 x REE and glycerol (p < 0.05). There was no difference in leucine oxidation between the dogs given triacetin at 1.0 x REE and control groups, whereas leucine oxidation decreased by 53% during triacetin infusion at 1.5 x REE (p < 0.05). Nonoxidative leucine disappearance, an indicator of protein synthesis, did not change in any of the studies.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
...Triacetin is more rapidly absorbed from the gastrointestinal tract in 3 hours than the other fats tested. Triacetin has been shown to be a source of liver glycogen and when fed in amounts equal in caloric value to 15% glucose it was utilized as efficiently as was glucose.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
杂种狗被用来确定在灌注5% v/v三醋酸水溶液期间,系统性的、后肢的、肠道的、肝脏的和肾脏的醋酸摄取量。在10只动物中,以[1-(14)C]-醋酸进行了7小时的启动连续灌注。在示踪剂灌注开始后3小时,动物以47微摩尔/千克/分钟的速度接受三醋酸灌注,持续4小时。在最后30分钟内,每隔15分钟采集一次血液和呼吸样本。在血浆醋酸浓度和特定活性以及呼出的[(14)-C02]中达到了稳态条件。主动脉、肾静脉、门静脉、股静脉和肝静脉中的血浆醋酸浓度分别为1180、935、817、752和473微摩尔/升(所有数值均近似)。在灌注三醋酸期间,醋酸转化率为2214微摩尔/分钟;系统性的醋酸转化率占三醋酸衍生醋酸的68%。
Mongrel dogs /were used/ to determine the systemic, hindlimb, gut, hepatic, and renal uptake of acetate during infusion of a 5% v/v aqueous solution of triacetin. A primed, continuous infusion of [1-(14)C]-acetate was continued for 7 hr with 10 animals. Three hours after the start of the tracer infusion, the animals were infused with triacetin at a rate of 47 umol/kg/min for 4 hr. Blood and breath samples were taken at 15-min intervals for the last 30 min. Steady-state conditions were achieved in plasma acetate concentrations and specific activity and in expired [(14)-C02]. Plasma acetate concentrations were 1180, 935, 817, 752, and 473 umol/L /(all values approximate)/ in the aorta, renal vein, portal vein, femoral vein, and hepatic vein, respectively. The acetate turnover rate during triacetin infusion was 2214 umol/min; systemic acetate turnover accounted for 68% of triacetin-derived acetate.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
TSCA:Yes
-
安全说明:S23,S24/25
-
WGK Germany:1
-
海关编码:2915390090
-
危险品运输编号:NONH for all modes of transport
-
RTECS号:AK3675000
-
危险性防范说明:P261,P280,P301+P312,P302+P352,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:镀锌铁桶包装。应储存在阴凉干燥处,注意防晒和防火。
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 三乙酸甘油酯 |
| 化学品英文名称: | Glyceryl triacetate;Triacetyl glycerin |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 102-76-1 |
| 分子式: | C 9 H 14 O 6 |
| 分子量: | 218.23 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | |||
| 化学品名称:三乙酸甘油酯 | |||
|
| 第三部分:危险性概述 |
| 危险性类别: | |
| 侵入途径: | 吸入 食入 经皮吸收 |
| 健康危害: | 可能有刺激作用,一般认为,作为食品及工业接触中不存在卫生问题。 |
| 环境危害: | 对环境有危害。 |
| 燃爆危险: | 本品可燃。 |
| 第四部分:急救措施 |
| 皮肤接触: | 用肥皂水及清水彻底冲洗。就医。 |
| 眼睛接触: | 拉开眼睑,用流动清水冲洗15分钟。就医。 |
| 吸入: | 迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。如呼吸停止,立即进行人工呼吸。就医。 |
| 食入: | 误服者,饮适量温水,催吐。就医。 |
| 第五部分:消防措施 |
| 危险特性: | 遇高热、明火或氧化剂,有引起燃烧的危险。 |
| 有害燃烧产物: | 一氧化碳、二氧化碳 |
| 灭火方法及灭火剂: | 尽可能将容器从火场移至空旷处。喷水保持火场容器冷却,直至灭火结束。处在火场中的容器若已变色或从安全泄压装置中产生声音,必须马上撤离。灭火剂:雾状水、泡沫、干粉、二氧化碳、砂土。 |
| 消防员的个体防护: | |
| 禁止使用的灭火剂: | |
| 闪点(℃): | 148 |
| 自燃温度(℃): | 433 |
| 爆炸下限[%(V/V)]: | |
| 爆炸上限[%(V/V)]: | |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 疏散泄漏污染区人员至安全区,禁止无关人员进入污染区,建议应急处理人员戴好口罩、护目镜,穿工作服。用大量水冲洗,经稀释的污水放入废水系统。如大量泄漏,利用围堤收容,然后收集、转移、回收或无害处理后废弃。 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | 密闭操作,全面通风。防止蒸气泄漏到工作场所空气中。操作人员必须经过专门培训,严格遵守操作规程。建议操作人员佩戴自吸过滤式防毒面具(半面罩),戴化学安全防护眼镜,穿防静电工作服,戴防化学品手套。远离火种、热源,工作场所严禁吸烟。使用防爆型的通风系统和设备。在清除液体和蒸气前不能进行焊接、切割等作业。避免产生烟雾。避免与氧化剂接触。配备相应品种和数量的消防器材及泄漏应急处理设备。倒空的容器可能残留有害物。 |
| 储存注意事项: | 储存于阴凉、通风的库房。远离火种、热源。防止阳光直射。保持容器密封。应与氧化剂分开存放,切忌混储。配备相应品种和数量的消防器材。储区应备有泄漏应急处理设备和合适的收容材料。 |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 中 国 MAC:未制订标准前苏联 MAC:未制订标准美国TLV—TWA:未制订标准 |
| 监测方法: | |
| 工程控制: | 生产过程密闭,全面通风。 |
| 呼吸系统防护: | 作业工人应戴口罩。高浓度环境中,建议佩戴防毒面具。 |
| 眼睛防护: | 一般不需特殊防护。 |
| 身体防护: | 穿工作服。 |
| 手防护: | 戴防护手套。 |
| 其他防护: | 工作时不得进食、饮水或吸烟。工作完毕,彻底清洗。保持良好的卫生习惯。 |
| 第九部分:理化特性 |
| 外观与性状: | 无色油状液体,稍有脂肪味。 |
| pH: | |
| 熔点(℃): | 4.1 |
| 沸点(℃): | 258~260 |
| 相对密度(水=1): | 1.164 |
| 相对蒸气密度(空气=1): | 7.52 |
| 饱和蒸气压(kPa): | 0.133/100℃ |
| 燃烧热(kJ/mol): | |
| 临界温度(℃): | |
| 临界压力(MPa): | |
| 辛醇/水分配系数的对数值: | |
| 闪点(℃): | 148 |
| 引燃温度(℃): | 433 |
| 爆炸上限%(V/V): | |
| 爆炸下限%(V/V): | |
| 分子式: | C 9 H 14 O 6 |
| 分子量: | 218.23 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 溶于水,可混溶于醇、醚、氯仿、苯。 |
| 主要用途: | 用作制造食品、肥皂、蜡烛、粘胶等的原料,也用作火药、皮革鞣制和染料的溶剂和增塑剂。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 稳定 |
| 禁配物: | 强氧化剂。 |
| 避免接触的条件: | |
| 聚合危害: | 不能出现 |
| 分解产物: | 一氧化碳、二氧化碳。 |
| 第十一部分:毒理学资料 |
| 急性毒性: | 属低毒类 LD50:3000mg/kg(大鼠经口),3250mg/kg(小鼠皮下) LC50: |
| 急性中毒: | |
| 慢性中毒: | |
| 亚急性和慢性毒性: | |
| 刺激性: | 家兔经眼: 116mg,引起刺激。 |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | |
| 生物降解性: | |
| 非生物降解性: | |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | |
| UN编号: | |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | |
| 运输注意事项: | 储存于阴凉、通风仓间内。远离火种、热源。保持容器密封。防潮、防晒。应与氧化剂分开存放。搬运时要轻装轻卸,防止包装及容器损坏。 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | 化学危险物品安全管理条例 (1987年2月17日国务院发布),化学危险物品安全管理条例实施细则 (化劳发[1992] 677号),工作场所安全使用化学品规定 ([1996]劳部发423号)等法规,针对化学危险品的安全使用、生产、储存、运输、装卸等方面均作了相应规定。 |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | 1.周国泰,化学危险品安全技术全书,化学工业出版社,1997 2.国家环保局有毒化学品管理办公室、北京化工研究院合编,化学品毒性法规环境数据手册,中国环境科学出版社.1992 3.Canadian Centre for Occupational Health and Safety,CHEMINFO Database.1998 4.Canadian Centre for Occupational Health and Safety, RTECS Database, 1989 |
| 填表时间: | 年月日 |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | 6 |
| MSDS修改日期: | 年月日 |
制备方法与用途
性质
无色无嗅的油状液体。与乙醇、乙醚、苯、氯仿等多数有机溶剂混溶,溶于丙酮,不溶于矿物油。稍溶于水,在25℃时水中溶解度为5.9g/100ml。能溶于二硫化碳、四氯化碳和苯甲酰氯,并在潮湿空气中水解成磷酸和氯化氢,产生白烟及特殊的刺激味。
用途三乙酸甘油酯可用作增塑剂及香料固定剂、油墨溶剂,亦用于医药和染料的合成。此外,它还用作色谱固定液、溶剂、增韧剂及香料固定剂,也可作为保湿剂、载体溶剂、增塑剂以及从天然气体中吸收二氧化碳。按我国GB 2760-96规定,可用于香料。
含量分析准确称取约1g试样放入耐压瓶中,加入1mol/L氢氧化钾溶液25.0ml和异丙醇15ml,加塞后包裹放于98℃±2℃水浴中加热1小时。取出瓶子,在空气中冷却至室温,松开包布和瓶塞释放余压,然后拆掉包布。加入酚酞指示剂6~8滴,用0.5mol/L硫酸滴定过量的碱至粉红色刚刚消失。空白试验同时进行。
每毫升0.5mol/L硫酸相当于三乙酸甘油酯(C9H14O6)36.37mg。
毒性ADI不作特殊规定(FAO/WHO,2001);GRAS(FDA,§182.1901,2000);LD50为3000mg/kg(大鼠,经口)。
使用限量FEMA(mg/kg):软饮料190;冷饮60~2000;糖果560;焙烤制品1000;胶姆糖4100。
食品添加剂最大允许使用量与残留标准 生产方法由醋酐与热甘油酯化后经真空蒸馏提纯而得;或由甘油与乙酸酯化制备。将甘油预热至50-60℃,加入乙酸、苯和硫酸,在加热搅拌下回流脱水,完成后回收苯,再加乙酐,加热4小时。冷却后用5%碳酸钠中和至pH7,分去水层,粗油经氯化钙干燥后减压蒸馏,收集128-131℃(0.93kPa)馏分为甘油三乙酸酯。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,2,3-丙三醇二乙酸酯 diacetin 102-62-5 C7H12O5 176.169 甘油三丁酸酯 tributyrin 60-01-5 C15H26O6 302.368 1,2-丙二醇二乙酸酯 1,2-diacetoxypropane 623-84-7 C7H12O4 160.17 (3-乙酰氧基-2-羟基丙基)乙酸酯 2-hydroxypropane-1,3-diyl diacetate 105-70-4 C7H12O5 176.169 甘油三甲酸酯 glycerol trimethanoate 32765-69-8 C6H8O6 176.126 甘油一乙酸酯 Glycerin-monoacetat 106-61-6 C5H10O4 134.132 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,2,3-丙三醇二乙酸酯 diacetin 102-62-5 C7H12O5 176.169 (3-乙酰氧基-2-羟基丙基)乙酸酯 2-hydroxypropane-1,3-diyl diacetate 105-70-4 C7H12O5 176.169 三硬脂酸甘油酯 glycerol tristearate 555-43-1 C57H110O6 891.497 甘油三甲基丙烯酸酯 glyceryl tri(methyl)acrylate 7401-88-9 C15H20O6 296.32 —— glycerol 1-acetate 2,3-carbonate 1607-31-4 C6H8O5 160.127 甘油一乙酸酯 Glycerin-monoacetat 106-61-6 C5H10O4 134.132 —— tri-γ-linolenin 14465-68-0 C57H92O6 873.354 —— D,L-glyceraldehyde diacetate 92175-31-0 C7H10O5 174.153 —— 12-acetyloxy-octadecanoic acid 2,3-bis-acetyloxy-propyl ester 330198-91-9 C27H48O8 500.673 —— 12-octadecanoyloxy-octadecanoic acid 2,3-diacetoxy-propyl ester 1037297-22-5 C43H80O8 725.103 —— 1,2,3-propanetriol 2-acetate 100-78-7 C5H10O4 134.132 3-氯-1,2-二乙酰氧基丙烷 1,2-diacetoxy-3-chloro-propane 869-50-1 C7H11ClO4 194.615 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:Ott, Chemische Berichte, 1914, vol. 47, p. 2391摘要:DOI:
-
作为产物:描述:参考文献:名称:C-2 甘油基化学:脂质损伤新机制的适应症摘要:合成了选择性生成 C-2 甘油自由基的前体,并通过 ESR 光谱、产物分析和动力学测量研究了相应自由基的化学行为。发现如果羟基存在于自由基碳中心,β-C,O 键的断裂会迅速进行。从自由基 30 中消除 β-乙酰氧基的速率常数取决于溶剂(甲醇中 kE = 4 × 105 s-1,甲苯中 kE = 2 × 107 s-1)。根据这些结果和从头计算,建议使用协同消除机制。α-甲氧基取代的 C-2 甘油基 42 和 43 在自由基阳离子的形成下表现出异裂 β-C,O 键裂解。对于酯取代的基团 24 和 35,没有观察到消除。DOI:10.1021/ja9641416
-
作为试剂:描述:3-溴-3-甲基丁-1-烯 在 三醋精 、 vanadium dioxide 作用下, 反应 2.16h, 以96%的产率得到异丁酸参考文献:名称:一种有机中间体二甲基乙酸的合成方法摘要:一种有机中间体二甲基乙酸的合成方法,主要包括如下步骤:在反应容器中加入2mol的3‑溴‑异戊烯,4‑6mol的甘油三乙酸酯溶液,控制搅拌速度130‑170rpm,持续30‑50min,降低溶液温度至7‑10℃,然后加入3‑5mol的二氧化钒,反应60‑80min,然后分3‑5次加入1500ml的溴化钾溶液,每次时间间隔20‑30min,加完后降低溶液温度至3‑5℃,静置2‑4h,硫酸钠溶液洗涤3‑5次,3‑甲氧基丙腈溶液提取4‑6次,邻二溴苯溶液提取5‑8次,在邻氯苯胺溶液中重结晶,脱水剂脱水,得晶体二甲基乙酸。公开号:CN108238889A
文献信息
-
[EN] BICYCLIC ARYL SPHINGOSINE 1-PHOSPHATE ANALOGS<br/>[FR] ANALOGUES D’ARYLSPHINGOSINE-1-PHOSPHATE BICYCLIQUES申请人:BIOGEN IDEC INC公开号:WO2011017561A1公开(公告)日:2011-02-10Compounds that have agonist activity at one or more of the SlP receptors are provided. The compounds are sphingosine analogs that, after phosphorylation, can behave as agonists at SlP receptors.
-
[EN] SUBSTITUTED QUINAZOLINES AS FUNGICIDES<br/>[FR] QUINAZOLINES SUBSTITUÉES, UTILISÉES EN TANT QUE FONGICIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2010136475A1公开(公告)日:2010-12-02The present invention relates to a compound of formula (I) wherein wherein the substituents have the definitions as defined in claim 1or a salt or a N-oxide thereof, their use and methods for the control and/or prevention of microbial infection, particularly fungal infection, in plants and to processes for the preparation of these compounds.本发明涉及一种具有如下式(I)的化合物,其中取代基具有权利要求1中定义的定义,或其盐或N-氧化物,它们的用途以及用于控制和/或预防植物中微生物感染,特别是真菌感染的方法,以及制备这些化合物的方法。
-
[EN] MICROBIOCIDAL OXADIAZOLE DERIVATIVES<br/>[FR] DÉRIVÉS D'OXADIAZOLE MICROBIOCIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2017157962A1公开(公告)日:2017-09-21Compounds of the formula (I) wherein the substituents are as defined in claim 1, useful as a pesticides, especially fungicides.式(I)的化合物,其中取代基如权利要求1所定义,作为杀虫剂特别是杀菌剂有用。
-
CHIMERIC MELANOCORTIN LIGANDS AND METHODS OF USE THEREOF申请人:REGENTS OF THE UNIVERSITY OF MINNESOTA公开号:US20180118789A1公开(公告)日:2018-05-03The invention provides compounds having the general formula I: and salts thereof, wherein the variables Pro, DPro, DPhe, Arg, Trp, X 1 , X 2 , X 3 and X 4 have the meaning as described herein, and compositions containing such compounds and methods for using such compounds and compositions.这项发明提供了具有一般式I的化合物及其盐,其中变量Pro、DPro、DPhe、Arg、Trp、X1、X2、X3和X4的含义如本文所述,并包含这种化合物的组合物以及使用这种化合物和组合物的方法。
-
[EN] COMPOSITIONS AND METHODS FOR THE TREATMENT OF ATHEROTHROMBOSIS<br/>[FR] COMPOSITIONS ET PROCÉDÉS POUR LE TRAITEMENT DE L'ATHÉROTROMBOSE申请人:KANDULA MAHESH公开号:WO2013024376A1公开(公告)日:2013-02-21The disclosures herein provide compounds of formula I or its pharmaceutical acceptable salts, as well as polymorphs, enantiomers, stereoisomers, solvates, and hydrates thereof. These salts may be formulated as pharmaceutical compositions. The pharmaceutical compositions may be formulated for peroral administration- transdermal administration, transmucosal, syrups, topical, extended release, sustained release, or injection. Such compositions may foe used to treatment of vascular disorders or conditions such as thrombotic cerebrovascular or cardiovascular disease or its associated complications.本公开提供公式I的化合物或其药用可接受的盐,以及其多晶型、对映体、立体异构体、溶剂合物和水合物。这些盐可以制成药物组合物。药物组合物可以用于口服、经皮、经粘膜、糖浆、局部、延长释放、持续释放或注射的给药。这种组合物可用于治疗血管疾病或病况,如血栓性脑血管或心血管疾病或其相关并发症。
表征谱图
-
氢谱1HNMR
-
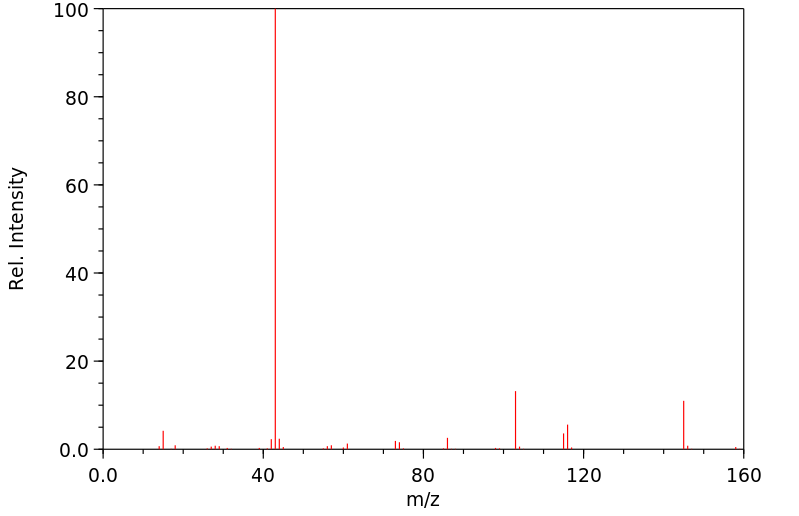
质谱MS
-
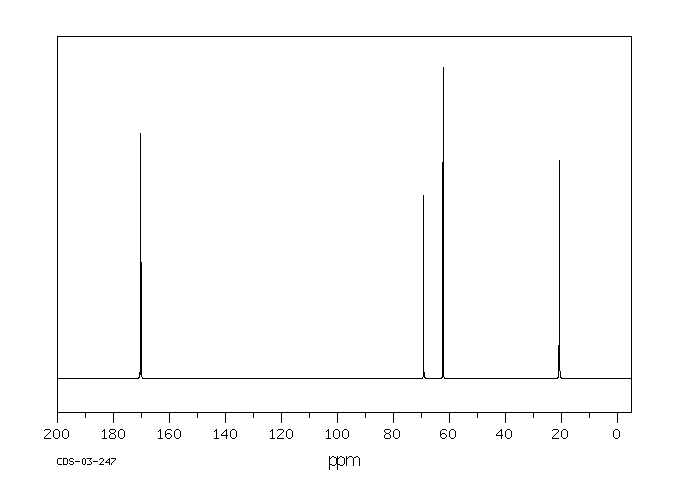
碳谱13CNMR
-
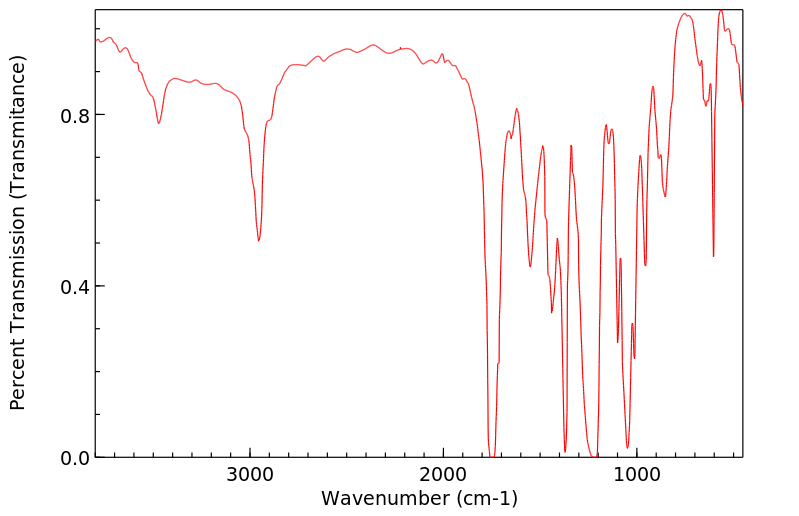
红外IR
-
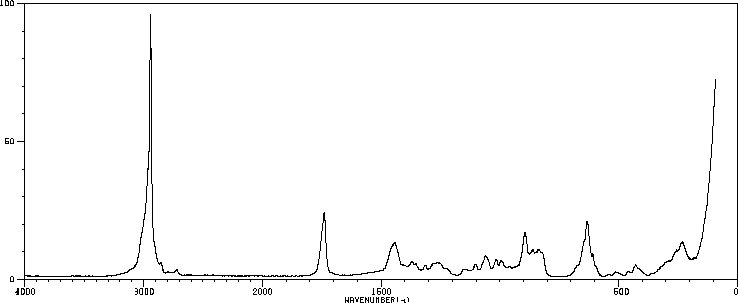
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-烯丙氧基-1,2-丙二醇
鲸蜡氧丙基甘油基甲氧丙基肉豆蔻酰胺
鲨肝醇硬脂酸酯
鲨肝醇
鲛肝醇
鲛肝醇
马来酸蓖麻油酯
辛氧基甘油
辛、癸酸甘油酯
甘油单肉豆寇酸酯
蓖麻油
苯丁酸甘油酯
苄氧基甲基-24-冠-8
肉豆蔻脑酸甘油酯
聚甘油基-2三异硬脂酸盐
聚甘油-6二硬脂酸酯
聚甘油-6三硬脂酸酯
聚甘油-4硬脂酸酯
聚甘油-3癸酸酯
聚甘油-3油酸酯
聚甘油-3二油酸酯
聚甘油-2硬脂酸酯
聚甘油-2油醚
聚甘油-2油酸酯
聚甘油-2月桂酸酯
聚甘油-2异硬脂酸酯
聚甘油-2倍半辛酸酯
聚甘油-2二油酸酯
聚甘油-2 癸酸酯
聚甘油-10油酸酯
聚甘油-10五硬脂酸酯
聚甘油-10二油酸酯
缩水甘油醚
神经酸甘油单酯
碳酸二(1-异丙氧基甲基-2-异丙氧乙基)酯
甲基3-[3-(叔-丁氧基羰基氨基)吖丁啶-1-基]-2-甲基-丙酸酯
甘油褐煤酸酯
甘油葡糖苷
甘油花生酸酯
甘油花生四烯酸酯
甘油芥酸酯
甘油聚醚-7三乙酸酯
甘油聚醚-26
甘油硫代乙醇酸酯
甘油烯丙基醚
甘油棕榈酸酯
甘油庚酸酯
甘油巯基丙酸酯
甘油基-1,2-二亚油酸酯-3-油酸酯
甘油基-1,2,3-三乙酰乙酸酯