丙炔胺盐酸盐 | 15430-52-1
物质功能分类
中文名称
丙炔胺盐酸盐
中文别名
炔丙基胺盐酸盐;2-丙炔胺盐酸盐;炔丙胺盐酸盐;3-氨基-1-丙炔盐酸盐
英文名称
prop-2-yn-1-amine hydrochloride
英文别名
propargylamine hydrochloride;mono-propargylamine hydrochloride;Hydron;prop-2-yn-1-amine;chloride
CAS
15430-52-1
化学式
C3H6N*Cl
mdl
MFCD00012907
分子量
91.5403
InChiKey
IKXNIQJDNKPPCH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:179-182 °C(lit.)
-
沸点:27-28 °C(lit.)
-
密度:0.989 g/mL at 25 °C(lit.)
-
闪点:91 °F
-
溶解度:甲醇(微溶)、水(微溶)
-
稳定性/保质期:
在常温常压下稳定,需避免以下物料:氧化物、酸、酸性氯化物、酸酐及热环境。
计算性质
-
辛醇/水分配系数(LogP):-0.4
-
重原子数:5
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:27.6
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险等级:6.1
-
危险品标志:T
-
安全说明:S26,S36/37/39,S45
-
危险类别码:R36/37/38,R25
-
WGK Germany:3
-
RTECS号:UK5076000
-
海关编码:2921199090
-
包装等级:II
-
危险类别:6.1
-
危险品运输编号:UN 2811 6.1/PG 2
-
危险性防范说明:P261,P301+P310,P305+P351+P338
-
危险性描述:H301,H315,H319,H335
-
储存条件:存储在0°C以下的密闭、阴凉干燥处。
SDS
炔丙胺盐酸盐 修改号码:5
模块 1. 化学品
产品名称: Propargylamine Hydrochloride
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第3级
急性毒性(经皮) 第3级
急性毒性(吸入) 第3级
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 吸入或皮肤接触或吞咽会中毒。
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 避免吸入。
只能在室外或通风良好的环境下使用。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
炔丙胺盐酸盐 修改号码:5
模块 2. 危险性概述
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。呼叫解毒中心/医生。
食入:立即呼叫解毒中心/医生。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
立即去除/脱掉所有被污染的衣物。
被污染的衣物清洗后方可重新使用。
若感不适:呼叫解毒中心/医生。
[储存] 存放于通风良好处。保持容器密闭。
存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 炔丙胺盐酸盐
百分比: >98.0%(N)(T)
CAS编码: 15430-52-1
俗名: Propynylamine Hydrochloride , 3-Amino-1-propyne Hydrochloride
分子式:
C3H5N·HCl
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
呼叫解毒中心/医生。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 立即呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(针对有毒颗粒的P3过滤式空气呼吸器)。远离溢出物/泄露
紧急措施: 处并处在上风处。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
炔丙胺盐酸盐 修改号码:5
模块 7. 操作处置与储存
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果可能,使用封闭系统。如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防尘面具,自携式呼吸器(SCBA),供气呼吸器等。使用通过政府标准的呼吸器。依
据当地和政府法规。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点: 180°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 溶于
[其他溶剂]
溶于: 乙醇
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 氯化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
炔丙胺盐酸盐 修改号码:5
模块 11. 毒理学信息
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第1项 毒害品。
UN编号: 2811
正式运输名称: 有毒固体, 有机物, 不另作详细说明
包装等级: III
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Propargylamine Hydrochloride
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第3级
急性毒性(经皮) 第3级
急性毒性(吸入) 第3级
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 吸入或皮肤接触或吞咽会中毒。
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 避免吸入。
只能在室外或通风良好的环境下使用。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
炔丙胺盐酸盐 修改号码:5
模块 2. 危险性概述
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。呼叫解毒中心/医生。
食入:立即呼叫解毒中心/医生。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
立即去除/脱掉所有被污染的衣物。
被污染的衣物清洗后方可重新使用。
若感不适:呼叫解毒中心/医生。
[储存] 存放于通风良好处。保持容器密闭。
存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 炔丙胺盐酸盐
百分比: >98.0%(N)(T)
CAS编码: 15430-52-1
俗名: Propynylamine Hydrochloride , 3-Amino-1-propyne Hydrochloride
分子式:
C3H5N·HCl
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
呼叫解毒中心/医生。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 立即呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(针对有毒颗粒的P3过滤式空气呼吸器)。远离溢出物/泄露
紧急措施: 处并处在上风处。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
炔丙胺盐酸盐 修改号码:5
模块 7. 操作处置与储存
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果可能,使用封闭系统。如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防尘面具,自携式呼吸器(SCBA),供气呼吸器等。使用通过政府标准的呼吸器。依
据当地和政府法规。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点: 180°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 溶于
[其他溶剂]
溶于: 乙醇
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 氯化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
炔丙胺盐酸盐 修改号码:5
模块 11. 毒理学信息
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第1项 毒害品。
UN编号: 2811
正式运输名称: 有毒固体, 有机物, 不另作详细说明
包装等级: III
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
用途:用于制作医药中间体。
反应信息
-
作为反应物:参考文献:名称:Radical Reaction of Sodium Hypophosphite with Terminal Alkynes: Synthesis of 1,1-Bis-H-phosphinates摘要:[GRAPHICS]The room-temperature radical addition of sodium hypophosphite to terminal alkynes produces the previously unknown 1-alkyl-1,1-bis-H-phosphinates in moderate yield. The reaction is initiated by R3B and air and proceeds under mild conditions in an open container. The bissodium salts precipitate spontaneously from the reaction mixtures, thus providing a simple purification procedure and the opportunity for multigram synthesis. The 1,1-bis-H-phosphinate products are novel precursors of the biologically important 1,1-bisphosphonates.DOI:10.1021/ol052533o
-
作为产物:描述:N-Boc-氨基丙炔 在 硫酸 、 sodium chloride 作用下, 以 neat (no solvent) 为溶剂, 反应 1.0h, 以99%的产率得到丙炔胺盐酸盐参考文献:名称:通过异位生成氯化氢气体进行无溶剂 N-Boc 脱保护摘要:描述了一种有效、可扩展且可持续的方法,用于在无溶剂条件下使用低至接近化学计量的氯化氢气体对氨基甲酸叔丁酯 ( N -Boc) 保护基团进行定量脱保护。我们展示了在两室反应器中从氯化钠和硫酸异位生成氯化氢气体,介绍了一种用于控制和化学计量释放 HCl 气体的简单方法。无溶剂条件允许对多种N进行脱保护-Boc衍生物以定量产率获得盐酸盐。该程序消除了对任何后处理或纯化步骤的需要,为标准方法提供了一种简单的绿色替代方案。由于无溶剂、无水条件,该方法对酸敏感官能团具有高耐受性,并提供扩展的官能团正交性。DOI:10.1039/d1ob00728a
文献信息
-
[EN] CONTROLLED DRUG RELEASE FROM SOLID SUPPORTS<br/>[FR] SUPPORTS SOLIDES POUR LA LIBÉRATION CONTRÔLÉE DE MÉDICAMENTS申请人:PROLYNX LLC公开号:WO2011140392A1公开(公告)日:2011-11-10The invention relates to solid supports useful in medical applications that provide controlled release of drugs, such as peptides, nucleic acids and small molecules. The drugs are covalently coupled to the solid support through a linkage that releases the drug or a prodrug through controlled beta elimination.这项发明涉及在医疗应用中有用的固体支持物,可提供药物的控制释放,如肽、核酸和小分子。这些药物通过一个通过受控β消除释放药物或前药的连接与固体支持物共价偶联。
-
[EN] CONTROLLED RELEASE FROM MACROMOLECULAR CONJUGATES<br/>[FR] LIBÉRATION CONTRÔLÉE À PARTIR DE CONJUGUÉS MACROMOLÉCULAIRES申请人:PROLYNX LLC公开号:WO2011140393A1公开(公告)日:2011-11-10The invention relates to conjugates of macromolecular carriers and drugs comprising linkers that release the drug or a prodrug through rate-controlled beta-elimination, and methods of making and using the conjugates.这项发明涉及大分子载体和药物的共轭物,包括释放药物或前药的连接剂,通过速率控制的β-消除释放药物,以及制备和使用这些共轭物的方法。
-
The development of a nucleus staining fluorescent probe for dynamic mitosis imaging in live cells作者:Krishna Kanta Ghosh、Yun-Mi Jeong、Nam-Young Kang、JungYeol Lee、Wan Si Yan Diana、Jun-Young Kim、Jaeduk Yoo、Dohee Kim、Yun Kyung Kim、Young-Tae ChangDOI:10.1039/c5cc02295a日期:——A low-toxicity nucleus staining fluorescent probe, CDb12, was developed for real time mitosis imaging in live cells. CDb12 was identified by unbiased high-throughput imaging-based screening of new xanthone library (AX)....开发了一种低毒性核染色荧光探针CDb12,用于在活细胞中进行实时有丝分裂成像。CDb12是通过对新的蒽酮库(AX)进行无偏高通量成像筛选而鉴定的。
-
Efficient Total Synthesis of Marine Alkaloid (−)-Nakadomarin A作者:Bin Cheng、Fufang Wu、Xiaobao Yang、Yuedong Zhou、Xiaolong Wan、Hongbin ZhaiDOI:10.1002/chem.201102101日期:2011.11.4Z's top: For the total synthesis of the marine alkaloid (−)‐nakadomarin A, (−)‐camphorsulfonic acid assisted, Z‐selective, olefin RCM of a monoamine (less polar) rather than a diamine (more polar) was found to be highly practical for constructing the F ring. The ABCD‐tetracyclic core was assembled through a novel efficacious PtCl2‐promoted cascade reaction sequence (see scheme).
-
Synthesis and evaluation of N-((1-benzyl-1H-1,2,3-triazol-4-yl)methyl)nicotinamides as potential anticancer agents that inhibit tubulin polymerization作者:Ahmed Kamal、N.V. Subba Reddy、V. Lakshma Nayak、Narasimha Rao Bolla、A.V. Subba Rao、B. PrasadDOI:10.1016/j.bmc.2014.04.038日期:2014.7A series of N-((1-benzyl-1H-1,2,3-triazol-4-yl)methyl)nicotinamides (4) was synthesized and tested for their anticancer activity against a panel of 60 human cancer cell lines. Some of the representative compounds such as 4a, 4b, 4f, 4g, 4i and 4t were selected for the five dose study and amongst them 4g and 4i displayed significant anticancer activity with GI50 values ranging from 0.25 to 8.34 and合成了一系列N -((1-苄基-1 H -1,2,3-三唑-4-基)甲基)烟酰胺(4),并测试了它们对一组60种人类癌细胞系的抗癌活性。选择了一些代表性化合物,例如4a,4b,4f,4g,4i和4t进行五剂研究,其中4g和4i在GI 50下显示出显着的抗癌活性。值分别为0.25至8.34和1.42至5.86μM。细胞周期分析表明,这些化合物诱导了MCF-7细胞在G2 / M期的细胞周期停滞。该系列中最活泼的化合物4g还抑制微管蛋白聚合,IC 50值比E7010高1.93μM。此外,通过研究对caspase-9的影响,Hoechst染色和DNA片段分析的方法表明,这些化合物通过凋亡诱导细胞死亡。对接实验表明它们与微管蛋白相互作用并有效结合。总体而言,结果表明,N -((1-苄基-1 H -1,2,3-三唑-4-基)甲基)烟酰胺骨架通过抑制微管蛋白聚合而具有抗癌特性。
表征谱图
-
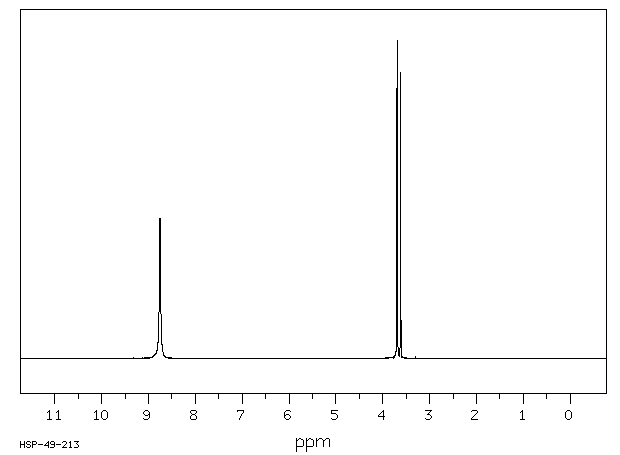
氢谱1HNMR
-
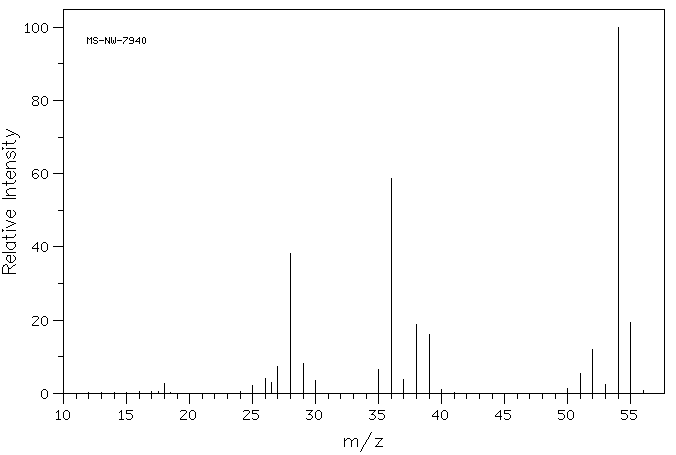
质谱MS
-
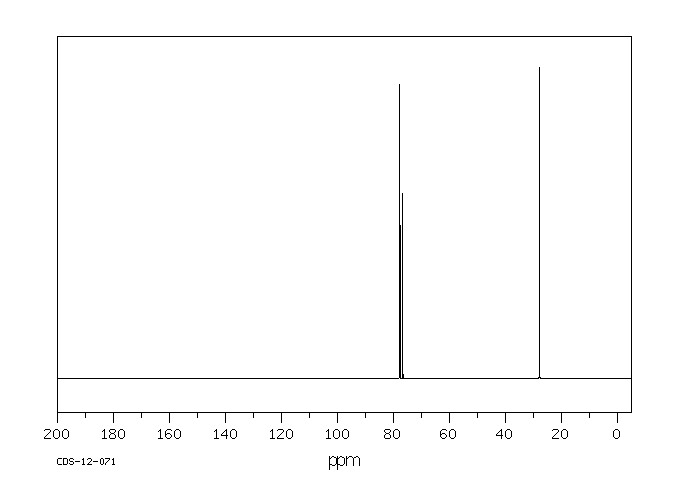
碳谱13CNMR
-
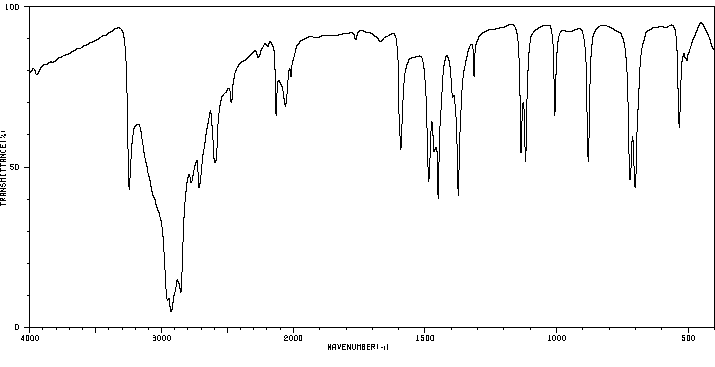
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
锗烷,三甲基[3-(三甲基甲锡烷基)-2-炔丙基]-
锗烷,三甲基-2-炔丙基-
铜,1-戊炔基-
甲基炔丙基硫化物
甲基乙炔和丙二烯混合物
甲基丙-2-炔基氰基二硫代亚氨酸酯
甲基-D3-乙炔
环戊基乙炔
环己基乙炔
环丙乙炔
炔丙胺
炔丙基膦
炔丙基碘化物
炔丙基叔丁基二甲基硅烷
炔丙基三甲基硅烷
炔丙基三乙基硅烷
氘乙炔
戊-1-炔-3-胺
戊-1,3-二炔
戊-1,2-二烯-4-炔
异氰基-乙炔
己基(己-5-炔基)甲基硅烷
己-1-炔银
四碳化铀
反式-4-(2-丙炔基)-环己烷甲醇
双(三甲基锡)乙炔
双(三氟甲基)锌
十四碳-1,4-二炔
十四碳-1,3-二炔
十八碳-1,17-二炔
十八炔
十三碳-1,7-二炔
十三碳-1,12-二炔
十一碳-1,5-二炔
亚硫酸二(2-丙炔基)酯
二甲基炔丙基溴化硫
二炔丙基硫醚
二乙炔基-二甲基-锗烷
二丙-1-炔基汞
二[2-甲氧基乙基汞(II)]乙炔
二(三正丁基甲锡烷基)乙炔
二(3-羟基-1-丙炔基)汞(II)
乙炔锂乙二胺配合物
乙炔银
乙炔基环己烷钠
乙炔基环丙烷氯化镁
乙炔基(三甲基)锗烷
乙炔基(三甲基)硅烷铜(1+)
乙炔基(三甲基)硅烷溴化镁
乙炔基(三甲基)硅烷氯化镁