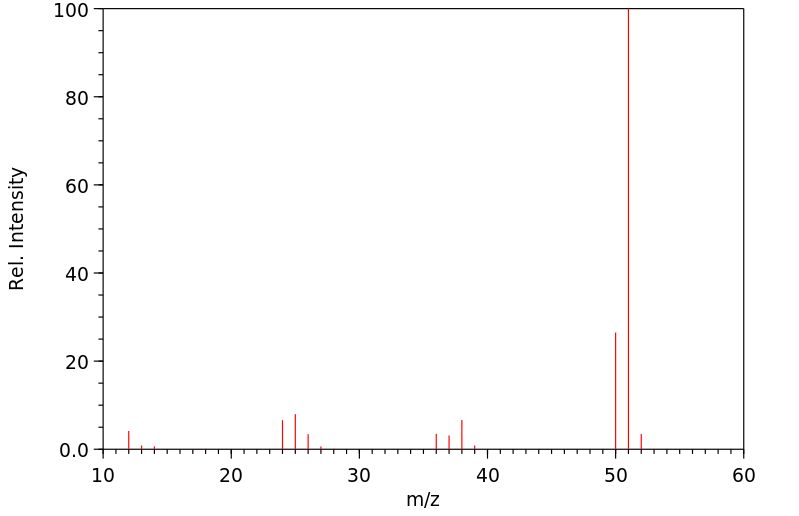
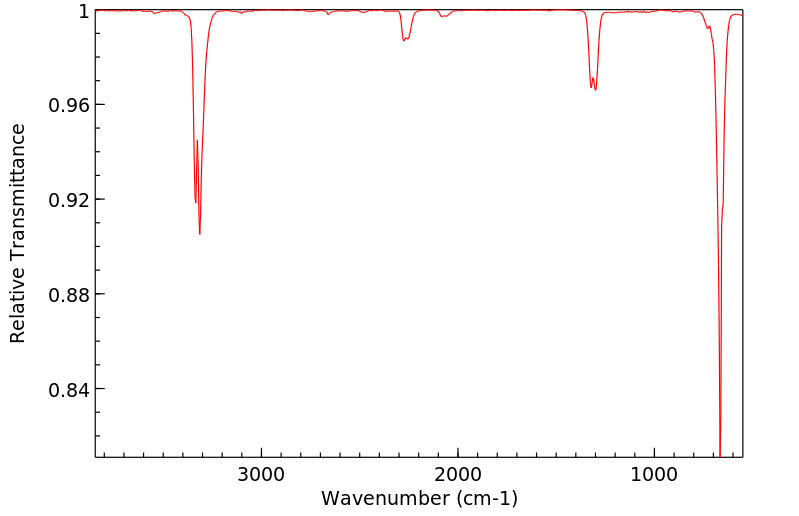
丙炔腈 | 1070-71-9
中文名称
丙炔腈
中文别名
5-溴-吡唑并[1,5-a]吡啶-3-羧酸乙酯;聚乙二醇十六烷基/油基醚
英文名称
propiolonitrile
英文别名
cyanoacetylene;monocyanoacetylene;prop-2-ynenitrile
CAS
1070-71-9
化学式
C3HN
mdl
MFCD01696133
分子量
51.0476
InChiKey
LNDJVIYUJOJFSO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:5℃
-
沸点:42.5℃
-
密度:0.8159 g/cm3 (17 ºC)
-
闪点:-30.5±12.6℃
-
保留指数:447.6
-
稳定性/保质期:
在溶液中易分解,并且非常敏感于空气和光线。
计算性质
-
辛醇/水分配系数(LogP):0.5
-
重原子数:4
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:23.8
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2926909090
SDS
制备方法与用途
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Murahashi et al., Nippon Kagaku Zasshi, 1956, vol. 77, p. 1689,1691摘要:DOI:
-
作为产物:描述:参考文献:名称:Rates of base-catalyzed hydrogen exchange of terminal acetylenes in aqueous solution. Absence of a resonance interaction摘要:DOI:10.1021/jo00356a011
-
作为试剂:参考文献:名称:水中核苷、核苷酸和甘油-3-磷酸胆碱的选择性酰化摘要:2',3'-二-O-乙酰基-核苷酸-5'-磷酸、2',3'-二-O-乙酰-核苷酸-5'-三磷酸和2',3',5的方便选择性合成'-tri-O-乙酰基-核苷在水中得到了开发。此外,还阐明了长链选择性甘油-3-磷酸胆碱二酰化。这些反应对环境无害、快速、收率高,并且产物易于纯化。重要的是,该反应可能表明关键代谢物的选择性酰化以促进它们并入原代谢中的益生元似是而非的反应途径。DOI:10.1055/s-0036-1588626
文献信息
-
Studies on Heteroaromaticity. XXI. 1,3-Dipolar Cycloaddition of<i>N</i>-Phenyl-<i>C</i>-(6-uracilyl)nitrone作者:Tadashi Sasaki、Moriyasu AndoDOI:10.1246/bcsj.41.2960日期:1968.12N-Phenyl-C-(6-uracilyl)nitrone (II) was prepared in 80% yield from orotaldehyde (I) and phenylhydroxylamine. II underwent 1,3-dipolar cycloaddition reactions with several ethylenic and acetylenic compounds to afford the corresponding isoxazolidines (IIIa—f) and isoxazolines (IVg—i). Similar reactions of II with phenyl isocyanate, carbon disulfide and with enamine were carried out successfully. The structural elucidation of these products was done on the basis of their NMR spectra.
-
Synthesis and biological activity of a new class of cytotoxic agents: N-(3-oxoprop-1-enyl)-substituted pyrimidines and purines作者:Francis Johnson、K. M. R. Pillai、Arthur P. Grollman、Lucy Tseng、Masaru TakeshitaDOI:10.1021/jm00374a004日期:1984.8thymine and adenine compounds are highly cytotoxic to a variety of tumor cell lines and inhibit macromolecular synthesis in cultured HeLa cells. Structure-activity studies, based primarily on the pyrimidine derivatives, reveal that the most potent inhibition occurs when the propenal group is located on the 3-nitrogen of a 2'-deoxyribonucleoside. The 3-(3-oxoprop-1-enyl) derivatives of thymidine, 2'-deoxyuridine合成了胸腺嘧啶和胞嘧啶的1-(3-氧代丙-1-烯基)衍生物以及腺嘌呤和鸟嘌呤的相应9-取代衍生物(博来霉素,Fe2 +和O2降解DNA的产物)并测试了其生物学活性。胸腺嘧啶和腺嘌呤化合物对多种肿瘤细胞系具有高度细胞毒性,并抑制培养的HeLa细胞中的大分子合成。主要基于嘧啶衍生物的结构活性研究表明,最有效的抑制作用发生在丙烯基位于2'-脱氧核糖核苷的3-氮原子上时。胸苷,2'-脱氧尿苷和5-碘-2'-脱氧尿苷的3-(3-氧代丙-1-烯基)衍生物有力且选择性地抑制了胸苷向DNA中的掺入(IC50大约等于0。5 microM)与用异氧尿苷观察到的结果相当。该系列中的活性化合物易于与含有伯氨基和巯基的亲核试剂反应。这项研究的结果为开发新型细胞毒剂提供了基础。
-
A convenient regioselective synthesis of cyclopentadienones via palladium-catalyzed [2+2+1] cyclocarbonylation of alkynes作者:Yanli Xu、Jinwu Zhao、Huoji Chen、Wanqing Wu、Huanfeng JiangDOI:10.1039/c3cc48308h日期:——A simple and efficient synthesis of cyclopentadienones via palladium-catalyzed cyclocarbonylation of alkynes under atmospheric pressure of carbon monoxide has been developed. The transformation was carried out under mild and ligand-free conditions, a wide range of substrates and exceptional functional group tolerance.
-
Synthesis and antimalarial activities of base-catalyzed adducts of 11-azaartemisinin作者:Belew Mekonnen、Everett Weiss、Esther Katz、Jingyuan Ma、Herman Ziffer、Dennis E KyleDOI:10.1016/s0968-0896(00)00049-3日期:2000.5to an amide nitrogen of olefins and terminal acetylenes conjugated with electron withdrawing groups (EWGs). When the terminal acetylene was conjugated with carbomethoxy, N,N-dimethyl amide or carbonyl groups, the E-adducts resulted. A mixture of E- and Z-adducts were obtained when the EWG was a nitrile. In vitro antimalarial activities of each compound were determined against two drug-resistant strains
-
The photomediated reaction of alkynes with cycloalkanes作者:Roísín A. Doohan、John J. Hannan、Niall W. A. GeraghtyDOI:10.1039/b517631j日期:——of a photomediator such as benzophenone, alkynes with electron-withdrawing groups react with cycloalkanes to give vinyl cycloalkanes. The reaction involves the regiospecific addition of a photochemically generated cycloalkyl radical to the beta-carbon of the alkyne. The stereochemical outcome of the reaction depends on the nature of the photomediator and alkyne used.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷