辛酸酐 | 623-66-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-1 °C
-
沸点:180 °C / 20mmHg
-
密度:0,91 g/cm3
-
保留指数:1878.3
-
稳定性/保质期:
远离强氧化剂和强碱。
计算性质
-
辛醇/水分配系数(LogP):5.9
-
重原子数:19
-
可旋转键数:14
-
环数:0.0
-
sp3杂化的碳原子比例:0.875
-
拓扑面积:43.4
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:8
-
安全说明:S26,S27,S36/37/39
-
危险类别码:R34
-
海关编码:2915900090
-
危险品运输编号:UN 3265
-
包装等级:II
-
危险类别:8
-
危险性防范说明:P234,P260,P264,P280,P301+P330+P331,P303+P361+P353,P304+P340,P305+P351+P338,P310,P321,P363,P390,P405,P406,P501
-
危险性描述:H290,H314
-
储存条件:存放在密封容器内,置于阴凉、干燥处。避免与火源及强氧化剂接触,并防止潮湿。
SDS
模块 1. 化学品
产品名称: n-Octanoic Anhydride
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
金属腐蚀性 第1级
健康危害
皮肤腐蚀/刺激 1B类
严重损伤/刺激眼睛 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 可能腐蚀金属
造成严重的皮肤灼伤和眼损伤
防范说明
[预防] 只可存放于原用的容器内。
切勿吸入。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。
食入:漱口。切勿催吐。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
被污染的衣物清洗后方可重新使用。
立即呼叫解毒中心/医生。
吸收溢出物,防止材料被损坏。
[储存] 存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
正辛酸酐 修改号码:5
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 正辛酸酐
百分比: >95.0%(GC)(T)
CAS编码: 623-66-5
分子式: C16H30O3
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
立即呼叫解毒中心/医生。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
立即呼叫解毒中心/医生。
食入: 立即呼叫解毒中心/医生。漱口。切勿引吐。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(自携式呼吸器)。远离溢出物/泄露处并处在上风处。确保
紧急措施: 足够通风。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
使用耐腐蚀设备。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放于惰性气体环境中。
防湿。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
潮敏
包装材料: 依据法律。只可存放在原用的容器內。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
正辛酸酐 修改号码:5
模块 8. 接触控制和个体防护
个人防护用品
呼吸系统防护: 半面罩或全面罩呼吸器,自携式呼吸器(SCBA),供气呼吸器等。依据当地和政府法
规,使用通过政府标准的呼吸器。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点:
-1°C
沸点/沸程 180 °C/2.7kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.91
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂, 强碱
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
正辛酸酐 修改号码:5
模块 12. 生态学信息
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第8类 腐蚀品
UN编号: 3265
正式运输名称: 腐蚀性液体, 酸性的, 有机的, 不另作详细说明
包装等级: II
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— formic octanoic anhydride 767292-01-3 C9H16O3 172.224 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 十二酸酐 lauric anhydride 645-66-9 C24H46O3 382.627 辛酸辛酯 octyl octylate 2306-88-9 C16H32O2 256.429 辛酸庚酯 2-heptyl octanoate 4265-97-8 C15H30O2 242.402 —— Octanoic acid 3-carboxy-propyl ester 30436-00-1 C12H22O4 230.304 辛酸甲酯 methyl octanate 111-11-5 C9H18O2 158.241 戊烷-3-基辛酸酯 1-Ethylpropyl octanoate 5457-67-0 C13H26O2 214.348 辛、癸酸甘油酯 monocaprylin 502-54-5 C11H22O4 218.293
反应信息
-
作为反应物:描述:辛酸酐 在 三(4-甲氧苯基)膦 、 甲基苯基硅烷 、 bis(dibenzylideneacetone)-palladium(0) 作用下, 以 甲苯 为溶剂, 反应 20.0h, 以93%的产率得到正辛醛参考文献:名称:钯催化的使用羧酸酐和氢硅烷的丙二烯的正式加氢酰化摘要:在市售的钯配合物作为催化剂的存在下,已经开发了使用羧酸酐作为酰基源和氢化硅烷作为还原剂的丙二烯的正式加氢酰化反应。该反应区域和立体选择性地提供α,β-不饱和酮。相似的催化剂体系对于使用氢化硅烷将羧酸酐还原为相应的醛也是有效的。DOI:10.1016/j.tet.2015.01.066
-
作为产物:参考文献:名称:一种由羧酸与三氯乙腈和三苯基膦合成对称酸酐的简便方法摘要:在室温下,在三乙胺的存在下,各种羧酸被转化为相应的羧酸酐,用三氯乙腈和三苯基膦处理。DOI:10.1081/scc-100000529
-
作为试剂:描述:参考文献:名称:Cameron et al., Journal of the Chemical Society, 1955, p. 2807,2811摘要:DOI:
文献信息
-
SYNTHETIC SUBSTRATES FOR ENZYMES THAT CATALYZE REACTIONS OF MODIFIED CYSTEINES AND RELATED METHODS申请人:The University of Chicago公开号:US20180147250A1公开(公告)日:2018-05-31Synthetic probes for detecting the activity of enzymes that catalyze reactions of post-translationally modified cysteine residues are described. The probes include “turn-on” probes that include a carbamate linkage that is cleaved via an intramolecular reaction with a free thiol produced by an enzyme catalyzed activity. The probes also include ratiometric, Michael addition-based probes that respond to enzymatic activity by a change in structure that results in a change in fluorescence properties. Methods of using the probes to detect enzymatic activity and disease are described. For example, the probes can be used to detect enzymatic activity in a variety of samples, including live cells and heterogeneous tissues. In addition, prodrugs that can be activated by enzymes that catalyze reactions of post-translationally modified cysteine residues and methods of using the prodrugs to treat disease are described.
-
Radical chlorinations of triglycerides作者:Philip E. Sonnet、Stanley OsmanDOI:10.1007/bf02541098日期:1995.3
Abstract Several triglycerides were synthesized with an iodoaroyl group. Intramolecular radical chain transfer chlorinations were conducted that resulted in the associated pair of fatty acids of the triglyceride becoming chlorinated. The distribution of monochlorinated species was similar to that obtained by direct radical chlorination of the relevant fatty acid methyl esters. Functionalization was, as expected, away from the carboxylate group but gave no indication that either alignment of chains or the constraints of an intramolecular process could limit the manifold of products of the reaction. To gauge the effect on halogenation of a structure, bearing more than one electron‐withdrawing group, methyl oleate was converted to the
bis trifluoroacetate of methyl 9,10‐dihydroxystearate. No products of chlorination on carbons 2–8 were observed. -
화장료 또는 피부외용제, 및 프로필 페닐 에테르 유도체申请人:SEIWA KASEI COMPANY LIMITED 세이와 카세이 콤파니 리미티드(520100108171)公开号:KR20150074160A公开(公告)日:2015-07-01본 발명은 우수한 멜라닌 생성 억제 효과(미백 효과), 항균 효과를 가짐과 동시에 경시적 안정성 등이 우수하여 화장료의 원료로서 바람직하게 사용되는 화합물로서, tert-부틸기 등이 치환된 페놀기의 수산기에, 수산기 등이 치환된 프로필기가 결합된 프로필 페닐 에테르 유도체 화합물을 제공하며, 또한 상기 화합물을 유효성분으로서 함유하는 멜라닌 생성 억제제, 미백제, 항균제 및 상기 화합물을 배합하는 것을 특징으로 하는 화장료를 제공한다.
-
[EN] ASPARAGINE DERIVATIVES AND METHODS OF USING SAME<br/>[FR] DÉRIVÉS D'ASPARAGINE ET LEURS PROCÉDÉS D'UTILISATION申请人:SENDA BIOSCIENCES INC公开号:WO2021252640A1公开(公告)日:2021-12-16The present disclosure relates to compounds of formulas (A) and (I), pharmaceutically acceptable salts thereof, and solvates of any of the foregoing, pharmaceutical compositions comprising the same, methods of preparing the same, intermediate compounds useful for preparing the same, and methods for treating or prophylaxis of diseases, in particular cancer, such as colorectal cancer, using the same.本公开涉及式(A)和(I)的化合物,其药学上可接受的盐,以及任何上述化合物的溶剂化合物,包括相同的药物组合物,制备相同的方法,用于制备相同的中间化合物,以及使用相同的方法治疗或预防疾病,特别是癌症,如结直肠癌。
-
One for Many: A Universal Reagent for Acylation Processes作者:Hyun Kyung Moon、Gi Hyeon Sung、Bo Ram Kim、Jong Keun Park、Yong-Jin Yoon、Hyo Jae YoonDOI:10.1002/adsc.201501177日期:2016.6.2This work describes acylation reactions facilitated by a type of heterocycle‐based acyl transfer agent, 2‐acyloxypyridazinone. Reactions of 2‐acyloxypyridazinone with carboxylic acids yield mixed carbonic anhydride intermediates, which are reactive and could be coupled with a wide range of substrates including acids, amines, alcohols, and thiols. The wide substrate scope, ease of operation (no additive
表征谱图
-
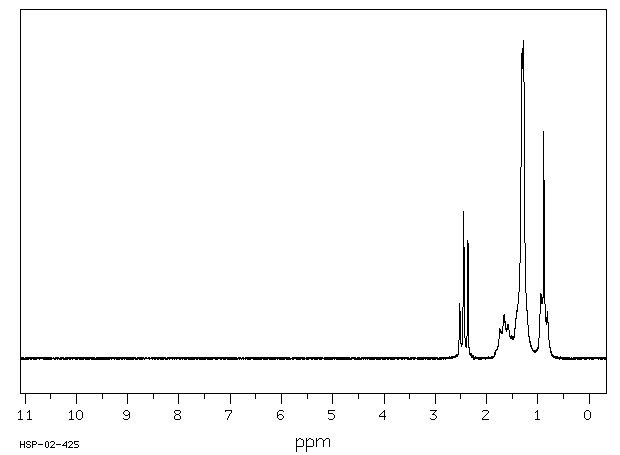
氢谱1HNMR
-
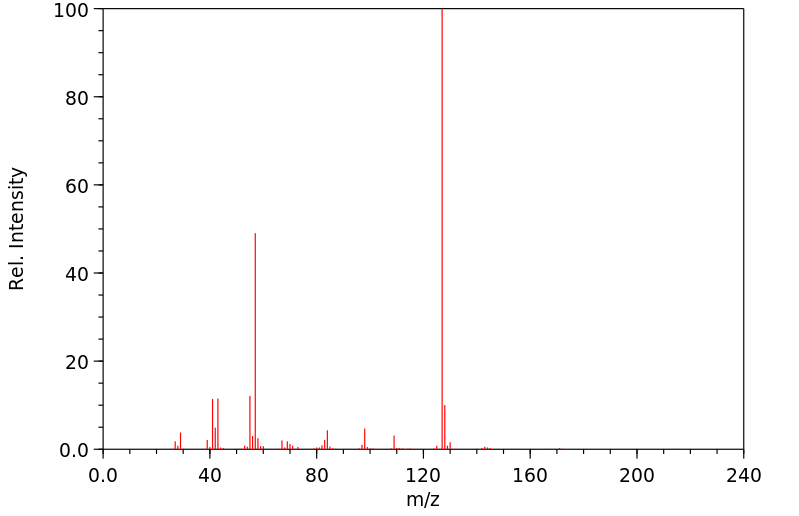
质谱MS
-
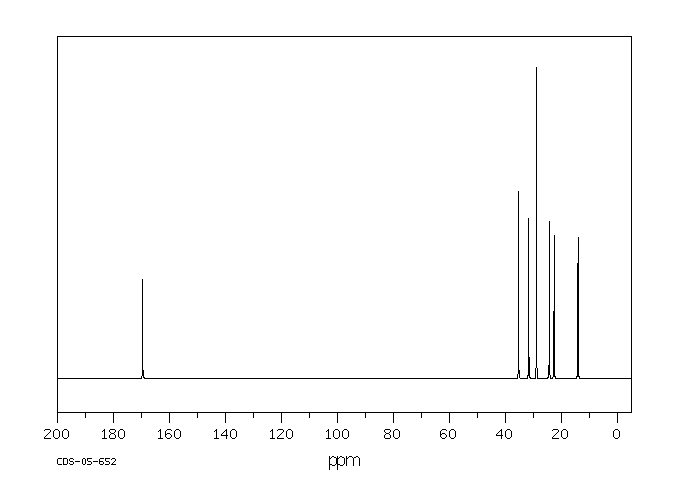
碳谱13CNMR
-
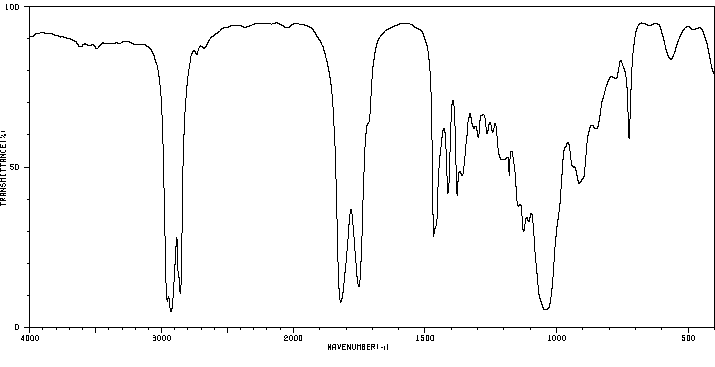
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息