二氯菊酸乙酯 | 59609-49-3
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:338.48°C (rough estimate)
-
密度:1.2191 (rough estimate)
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:14
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.7
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
SDS
| Name: | Ethyl 3-(2 2-dichlorovinyl)-2 2-dimethyl-1 Material Safety Data Sheet |
| Synonym: | |
| CAS: | 59609-49-3 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 59609-49-3 | Cyclopropanecarboxylic acid,3-(2,2-dic | 3-(2,2-D | 261-826-7 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
The toxicological properties of this material have not been investigated. Use appropriate procedures to prevent opportunities for direct contact with the skin or eyes and to prevent inhalation.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower lids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of soap and water for at least 15 minutes while removing contaminated clothing and shoes.
Remove contaminated clothing and shoes.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire.
Autoignition Temperature: Not available.
Flash Point: 110 deg C ( 230.00 deg F) NFPA Rating: Not published.
Explosion Limits, Lower: Not available.
Upper: Not available.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Keep container closed when not in use.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Personal Protective Equipment Eyes: Wear chemical goggles.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements must be followed whenever workplace conditions warrant a respirator's use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Not available.
Appearance: clear, colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Vapor Density: Not available.
Evaporation Rate: Not available.
Viscosity: Not available.
Boiling Point: 119.0 - 120.0 deg C @ 15.00mm Hg
Freezing/Melting Point: 0 deg C
Decomposition Temperature: Not available.
Solubility: Not available.
Specific Gravity/Density: 1.1170g/cm3
Molecular Formula: C10H14Cl2O2
Molecular Weight: 237.13
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Not available.
Hazardous Decomposition Products:
Irritating and toxic fumes and gases.
Hazardous Polymerization: Not available.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 59609-49-3 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Cyclopropanecarboxylic acid,3-(2,2-dichloroethenyl)-2,2-dimethyl-,ethylester - Not listed by ACGIH, IARC, NIOSH, NTP, or OSHA.
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
For further information, contact Fisher Scientific.
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
CDG/CPL
IMO
Not regulated as a hazardous material.
IATA
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Canadian TDG
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 59609-49-3:
Canada
CAS# 59609-49-3 is listed on Canada's DSL/NDSL List.
WHMIS: Not available.
CAS# 59609-49-3 is not listed on Canada's Ingredient Disclosure List.
Exposure Limits
US FEDERAL
TSCA
CAS# 59609-49-3 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
化学性质
二氯菊酸乙酯是一种无色透明液体。其沸点在101帕斯卡下为93~94℃,在27帕斯卡下的沸点为78~88℃。该化合物的折射率为1.4833,相对密度为1.1170。它不溶于水,但能溶解于苯、甲苯、氯仿等有机溶剂中。
用途
二氯菊酸乙酯是合成拟除虫菊酯类农药的重要中间体,可用于制备如氯菊酯、氯氰菊酯、高效氯氰菊酯、四氟苯菊酯和氟氯氰菊酯等多种农药。
生产方法
该化合物的制备方法包括以下几种:
-
Farks法(重氮乙酸酯法) 通过异丁烯与三氯乙醛在AlCl₃催化下缩合,再经乙酰化、锌粉还原和酸催化异构化得到共轭双烯,即1,1-二氯-4-甲基戊二烯-[1,3]。随后,在铜粉的催化作用下与重氮乙酸甲酯(或乙酯)反应生成二氯菊酸甲酯(或乙酯)。
-
相模法 由2-甲基丁烯[2]-醇[1](异戊烯醇)和原乙酸甲酯(或乙酯),在丙酸存在下的酸性条件下进行缩合Clasen重排,生成3,3-二甲基戊烯[4]酸甲(乙)酯。该化合物与四氯化碳在过氧化苯甲酰的催化下调聚加成,得3,3-二甲基-4,6,6,6-四氯己烯[5]酸甲(乙)酯。随后,在甲醇钠的作用下脱除氯化氢并环合而成二氯菊酸甲酯(或乙酯)。
-
相模-库拉莱法 由1,1,1-三氯-2-羟基-4-甲基戊烯[4]经对甲基苯磺酸催化异构化,得到1,1,1-三氯-2-羟基-4-甲基戊烯-[3]。再与原乙酸三甲(乙)酯进行缩合Clasen重排反应,生成3,3-二甲基-4,6,6-三氯己烯[4]酸甲(乙)酯。在环合过程中还生成部分的二氯丁内酯。3,3-二甲基-4,6,6-三氯己烯[4]酸甲(乙)酯再于乙醇钠存在下环合成二氯菊酸甲(乙)酯,而二氯丁内酯通过氯化氢或氯化亚砜在甲(乙)醇溶液中开环生成3,3-二甲基-4,6,6-三氯己烯[4]酸甲(乙)酯并环合成二氯菊酸甲(乙)酯。
-
用异戊二烯为原料的方法 首先通过与两分子氯化氢反应生成1,3-二氯-3-甲基丁烷,再与偏二氯乙烯反应生成1,1,5-三氯-3,3-二甲基环丁酮。在三乙胺的存在下经Cine重排得2-三氯乙基-3,3-二甲基-4-氯环丁酮,再进行Favorski重排得3-三氯乙基-2,2-二甲基环丙烷羧酸。最后通过碱处理脱去氯化氢即得到二氯菊酸。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 二氯菊酸 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylic acid 55701-05-8 C8H10Cl2O2 209.072 2-(2,2-二氯乙烯基)-3,3-二甲基-1-乙氧基羰基环丙烷羧酸乙酯 ethyl 2-(2,2-dichlorovinyl)-3,3-dimethyl-1-ethoxycarbonylcyclopropanecarboxylate 59609-50-6 C13H18Cl2O4 309.19 —— ethyl 3-formyl-2,2-dimethylcyclopropanecarboxylate 66692-75-9 C9H14O3 170.208 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 反式右旋菊酸(DV菊酸) (1R)-trans-3-(2,2-dichloro-1-ethenyl)-2,2-dimethylcyclopropanecarboxylic acid 55701-03-6 C8H10Cl2O2 209.072 —— (-)-cis-(1S,3S)-2,2-dimethyl-3-(2',2'-dichlorovinyl)cyclopropanecarboxylic acid 55701-08-1 C8H10Cl2O2 209.072 二氯菊酸 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylic acid 55701-05-8 C8H10Cl2O2 209.072 —— 2,2-dimethyl-3-(2,2-dibromovinyl)-cyclopropane-1-carboxylic acid ethylester 59898-05-4 C10H14Br2O2 326.028 氯菊酯 permethrin 52645-53-1 C21H20Cl2O3 391.294
反应信息
-
作为反应物:描述:参考文献:名称:Process for the preparation of dihalovinylcyclopropanecarboxylic acids摘要:本发明涉及一种制备环丙烷羧酸的新工艺,其中环丙烷羧酸的化学式为(I)##STR1## 其中X和Y独立地代表卤素,通过相转移催化剂存在下,在或不在水溶性有机溶剂中,通过相应的具有1到6个碳原子的烷基酯的碱性水解来实现。水解是在2至50重量%的碱性氢氧化物溶液中进行的。如果需要,在适当条件下,可以改变最终产物中原始酯的顺反异构比。化学式(I)的环丙烷羧酸以高纯度获得,并且是杀虫活性拟除虫菊酯的有用中间体。公开号:US04419524A1
-
作为产物:描述:3.3-二甲基-4.6,6-三氯己烯[5]酸乙酯 以78%的产率得到二氯菊酸乙酯参考文献:名称:Process for preparation of substituted cyclopropane carboxylic acids and摘要:当1-卤代-3-烯基-2-醇与邻位羧酸酯和/或酮缩醛反应时,主要反应产物是γ-卤代-δ-不饱和羧酸酯。当这种中间体与碱性物质反应时,形成取代环丙烷羧酸酯。这种酯可以直接用作杀虫剂或农药,也可以在将酯的醇残基转化为其他醇残基后使用。公开号:US04113968A1
文献信息
-
Production of insecticidally active vinyl-cyclopropane carboxylic acid申请人:Bayer Aktiengesellschaft公开号:US04291176A1公开(公告)日:1981-09-22Insecticidally active vinyl-cyclopropane carboxylic acid esters of the formula ##STR1## are prepared by reacting ##STR2## in which R.sup.12 is a radical selected from the group consisting of ##STR3## with an alcoholate of the formula M--O--R.sup.8. Various processes for making the intermediates are also described. Many of the intermediates and end products are new.
-
A Practical and Efficient Synthesis of Fluorinated Pyrethroids.<i>cis</i>-3-(2-Chloro-3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylates作者:Mayumi Nishida、Takamasa Fuchikami、Kiyosi KondoDOI:10.1246/cl.1994.703日期:1994.4A practical and efficient route to fluorinated pyrethroids has been developed which involves the selective formation of the cis cyclopropane ring using intramolecular alkylation of the haloaldehyde and a novel transformation of the aldehydes to the corresponding esters via the cyanohydrins.
-
Process for preparing dihalovinylcyclopropanecarboxylates申请人:Sagami Chemical Research Center公开号:US04214097A1公开(公告)日:1980-07-22Novel syntheses of dihalovinylcyclopropanecarboxylates, including potent insecticides, are described. The processes begin with the reaction between an alkenol and an orthoester to produce a .gamma.-unsaturated carboxylate, followed by the catalyzed addition of a carbon tetrahalide to the double bond and dehydrohalogenation to produce a cyclopropane derivative.
-
A novel chain reaction induced by cathodic reduction. Addition of trichloromethyl anion to aldehydes or vimyl acetate作者:Tatsuya Shono、Hiroshi Ohmizu、Souta Kawakami、Shinji Nakano、Naoki KiseDOI:10.1016/0040-4039(81)80018-4日期:1981.1A novel chain reaction induced by cathodic reduction was found in the reaction system consisting of carbon tetrachloride, chloroform, and electrophiles such as aldehydes or vinyl acetate. The current efficiency of addition of trichloromethyl anion to electrophiles was extremely high. Synthesis of an analogue of ethyl chrysanthemate using this new reaction was also described.
-
Transition metal catalyzed reactions of diazoesters作者:A.J. Anciaux、A. Demonceau、A.F. Noels、R. Warin、A.J. Hubert、P. TeyssiéDOI:10.1016/s0040-4020(01)91934-9日期:——of carbenes generated by catalyzed decomposition of diazoesters in the presence of Rh, Pd and Cu catalysts can be controlled to some extent by selecting proper reaction parameters. For a particular diene, the regioselectivity depends both on the catalyst and on the nature of the double-bond (conjugation, substitution).在Rh,Pd和Cu催化剂存在下,通过重氮催化催化重氮酸酯分解生成的卡宾的环加成反应,多烯环丙烷化的区域选择性可以在一定程度上控制。对于特定的二烯,区域选择性既取决于催化剂又取决于双键的性质(共轭,取代)。
表征谱图
-
氢谱1HNMR
-
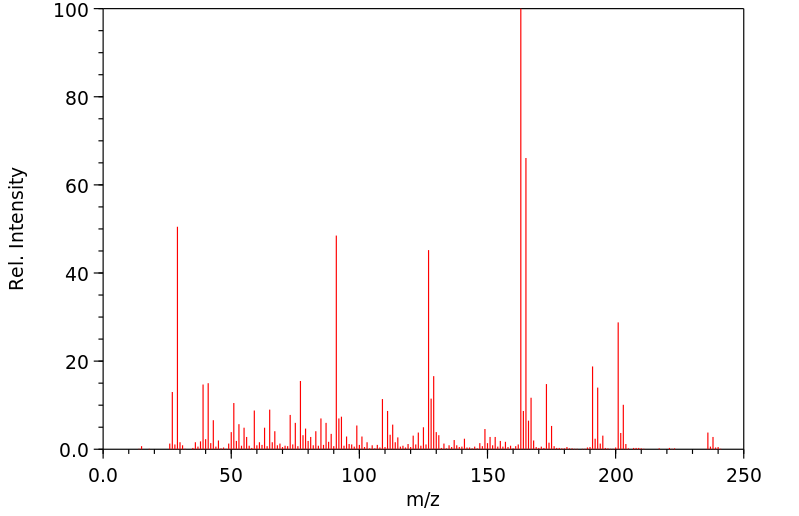
质谱MS
-
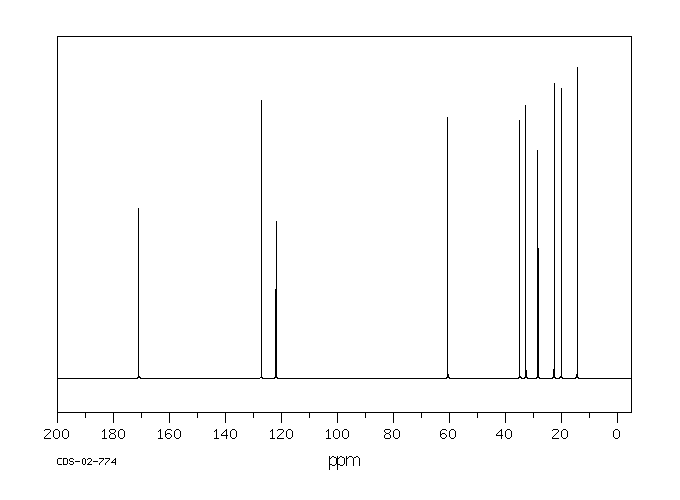
碳谱13CNMR
-
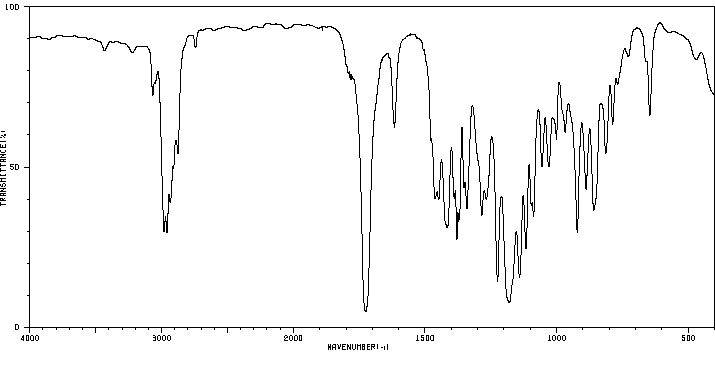
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息